Non-representative surveys in many countries have suggested that there is a high use of complementary and alternative medicine (CAM). CAMs include herbal medicines, traditional medicines (Ayurvedic or Chinese), vitamin, mineral and nutritional supplements, homeopathic medicines and aromatherapy products. CAM practices include a diverse group of therapies such as herbal medicine, chiropractic, osteopathy, naturopathy, homeopathy and acupuncture. In the United States, about a third of adults aged 18 years or older use CAMs.1
In Australia, we previously conducted two representative population surveys in South Australia examining the use and cost of CAMs, using the South Australian Health Omnibus Surveys. We found that, between 1995 and 2000, there had been an increase in the use of CAMs and over-the-counter medicines.2,3
In 2004, we conducted a third representative population survey in South Australia, surveying the trend in CAM use since the Pan Pharmaceutical crisis (in which a substantial quantity of CAM products was removed from retail stores) and asking further questions about the public’s beliefs concerning the CAMs and their quality of life.
Data were collected via the South Australian Health Omnibus Survey conducted in March–April 2004. The Health Omnibus Survey is a large representative population survey that has been undertaken annually in South Australia since 1990 using a clustered, multistage, systematic, random, self-weighting area sample. Weighting ensures that every household has the same probability of being selected. This approach has been used consistently from the inception of the survey and involves sampling people aged 15 years and over living in metropolitan Adelaide and major country towns with a population exceeding 1000.4 South Australia has a slightly older population on average than other Australian states, but otherwise the population is generally similar to Australian demographic data.
The survey data were weighted to the 2002 Australian Bureau of Statistics Estimated Residential Population data by sex, 5-year age groups and geographic area so that the findings apply to the demographic profile of South Australia. To estimate reliability, 10% of the respondents were resurveyed.
Age, sex, marital status, education, employment status, area of residence, country of birth, and household income level were recorded.
The respondents were given the following definition:
They were asked whether they had used any complementary or alternative medicines or health products over the past year, with seven main types listed on a prompt card with samples of each type (herbal medicines, vitamins, mineral supplements, Chinese medicines, homeopathic medicines, soy products, aromatherapy oils, other or none). Excluded were calcium, iron or vitamins prescribed by a medical practitioner. The respondents were asked to estimate, to the nearest dollar, the monthly cost of these products.
A prompt card was used to seek the main reasons for the use of these medicines, with the categories general health; blood or circulation; bladder or kidneys; muscles, bones or joints; lungs or sinuses; immune system; nerves or stress; stomach and bowels; prostate; PMS/menopause; skin; other; and don’t know.
Respondents were asked if their medical practitioner knew about the complementary medicines being taken and whether they used these medicines on the same day that they took conventional medicines.
They were also asked if they had visited any of the following therapists listed on a prompt card in the last year: herbal therapist/herbalist; naturopath/natural therapist; aromatherapist; homeopath; acupuncturist; iridologist; osteopath; chiropractor; reflexologist; other; none; and don’t know. The approximate total yearly cost of these therapists (not including the cost of any medicine they prescribed or sold) was asked.
Respondents were asked if any children in their household were ever given CAM medicines or therapies with the following options: no children in household; children but alternative medicine and therapies not used; yes — non-prescribed vitamins; yes — other alternative medicines/products; yes — therapists listed on the above prompt card; other alternative therapists; and don’t know.
Respondents were asked: “Do you think that complementary or alternative medicines are independently tested by a government agency such as the Therapeutic Goods Administration before being sold?” and “What do you think they are tested for?”. Possible answers were quality/safety/side effects; efficacy/strength/effect; that they do what they claim to do; other; and don’t know.
Similar questions, but not all, had been asked using the same method in surveys of the same population in 1993 and 2000.2,3 Where possible, the answers to the same questions were compared between surveys.
The data were analysed using SPSS version 12.0 (SPSS Inc, Chicago, Ill, USA) and Epi Info version 6.04 (Centers for Disease Control and Prevention, Atlanta, Ga, USA). All cost data were adjusted using the consumer price index.
The South Australian Health Omnibus Advisory Committee independently vetted all questions in the survey and gave its ethical approval.
In the 2004 representative population sample, 52.2% (n = 1574) of the sample said they had used a CAM over the past year. CAM use was greatest in women, individuals with post-secondary school education, in the 25–44-year age bracket, respondents with household income over $30 000, those who live in the metropolitan area, and in those who were born in Australia (Box 1). Lower use was reported for those older than 65 years, separated or divorced, with no post-secondary school education, and those who lived in a household with incomes totalling less than $30 000.
Respondents reported self-prescribed vitamins as the most used (39.2% of all respondents), followed by herbal medicines (20.6%) and mineral supplements (13.6%). Aromatherapy (11.2%) was the only other category mentioned by more than 5% of the sample (Box 2).
Longitudinal comparison with similar studies2,3 carried out in 1993 and 2000 showed a strong consistency among the total CAM use in South Australia (50% in 1993, 52.1% in 1993 and 52.2% in 2004) (Box 2). The use of herbal medicine has risen in both men and women, with women’s use increasing from 16.6% of female respondents in 2000 to 24.9% in 2004 (χ2 = 35.1; P < 0.01).
The primary reason for using CAMs was for general health (Box 3). The reasons for use of CAMs differed with age, marital status and education. For example, use of CAMs for blood or circulation and muscles, bones or joints increased with age, whereas use for the immune system decreased with age; those who had never married had a higher use of CAMs for the immune system (23.3%); those who were separated or divorced had higher use for nerves or stress (27.3%); and those who had completed a bachelor degree or higher had a higher use of CAM products for both general health (77.7%) and the immune system (25.7%), and a lower use for muscles, bones and joints (13.6%).
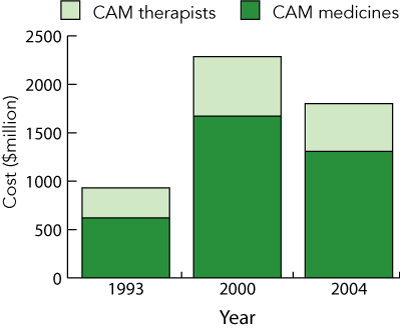
The mean expenditure reported by all CAM users on the cost of CAM per month was $21.23 (range, $1 to $650 per month). Women spent significantly more on CAM per month ($23.24) than men did ($18.50) (t = 2.5, df = 1248, P < 0.01). The extrapolated expenditure for the Australian public in 1993, 2000 and 2004 is shown in Box 4. There has been a reduction in total expenditure on CAMs since 2000, from $1671 million to $1308 million.
The use of CAM therapists in the three surveys is shown in Box 5. In the past year, 26.5% of respondents had visited at least one CAM therapist. Chiropractors were the most commonly used CAM therapist. Women (29.3%) used CAM therapists more often than men did (23.6%) (χ2 = 12.6, P < 0.01). With the exception of herbal therapists and “other”, there has been no escalation in the use of CAM therapists since 2000.
Use of CAM therapists peaked in the middle age brackets (35–44 years, 31.6%; 45–54 years, 32.6%) and tailed off at either end of the age spectrum (under 25 years, 25.8%; over 65 years, 16.5%). Use was higher in country areas (29.4%) than metropolitan areas (25.3%). High use of CAM therapists was reported by respondents born in Australia (28.7%). Low use was reported for those who left school before the age of 15 years (17.2%). Use also increased as household income increased, with individuals reporting an annual income up to $12 000 using them substantially less than those reporting an income greater than $80 000 per annum (17.2% compared with 31.5%; χ2 = 16.3, P < 0.01).
The mean annual expenditure on CAM therapists was AUD$225 (range, $5 to $5000 per year). The extrapolated Australian expenditure for CAM therapists and the total cost of CAM in 1993, 2000 and 2004 is shown in Box 4. The cost of CAM therapists in 2004 has decreased since 2000, from $616 million to $494 million.
This third representative population survey of CAM use shows that in 2004 half the South Australian population used CAMs and more than a quarter visited CAM therapists. This survey raises a number of interesting observations and important concerns, among them that most survey respondents incorrectly assumed that the TGA had audited the efficacy and safety of complementary medicines.
Although there were no significant changes in the numbers of respondents who use CAMs since 2000, in 2004 there was a significant increase in the number of women compared with men using herbal medicines. As confirmed in multivariate analyses in our previous surveys, the greatest users of CAMs are better-educated, higher income, women in the 25–44 years age group living in a metropolitan area. Self-prescribed vitamins were the most used products, followed by herbal medicines. Most respondents had no specific medical reason for using CAMs, but believed they would help their general health. Younger respondents were more li-able to believe that CAMs helped their immune system and older respondents used them more often for chronic disabilities such as joint pains or circulation problems. Women often try herbal medicines around their menopause.5 For the first time, we asked about children’s use of CAMs — almost a third of respondents with children had administered CAMs to their children.
In 2004, the overall extrapolated expenditure in Australia on complementary medicines from our data decreased to $1.31 billion from $1.67 billion in 2000. However, as there was little change in the numbers using CAMs and as, in general, there was little change in the cost of the commonly used products, it is likely that CAM users are now using fewer CAMs per person than in 2000.
This decline in the market could be contributed to consumer response to the adverse publicity in the media surrounding CAMs in 2003 during the Pan Pharmaceutical crisis. In 2003, the TGA had serious concerns about the content and quality control of products manufactured by Pan Pharmaceuticals. After an audit prompted by a range of hospitalisations and other serious adverse events to an over-the-counter non-CAM product manufactured by the company, the TGA suspended the company’s licence to manufacture, and required the recall of 1600 products — mostly CAMs. It has been estimated that this company manufactured about 40% of CAMs on the Australian market. The suspension of its licence resulted in a substantial quantity of CAMs being removed from retail outlets, which may have interrupted or reduced respondents’ use of CAMs.
A similar effect was seen in the use of menopausal hormone replacement therapies in 2002 when media reported, and often exaggerated, the risks of long-term therapy with one regimen.6 Hormone therapy use dropped to a third of its prior use, but has since returned to two-thirds of the levels of use recorded before 2002. Thus, the media appears to have a great influence on decision-making regarding both conventional and complementary medication.
In contrast to the decline in use of CAMs, there has been a steady increase in use of CAM therapists by the South Australian public over the past 10 years. Chiropractors, followed by naturopaths, were the most frequently visited. The profile of the most common user was a woman, between the ages of 35 and 44 years, living in the country, with a higher than average income level. There was a wide range of expenditure on CAM therapists but, again, overall average costs since 2000 had decreased (by 20%), suggesting a lower frequency of attendance.
It is of continuing concern that about half of CAM users ingested them on the same day as conventional medicines and that more than half (53.2%) did not tell the doctor prescribing conventional medicines that they were also taking CAMs. This is a critical issue that needs to be addressed to ensure that the use of CAMs by the community is consistent with the National Strategy for Quality Use of Medicines. Interactions between some CAMs and some prescribed medicines have been frequently described.7,8 Yet, lay beliefs are that most CAMs are safe.3 This is in contrast to increasing reports of adverse effects from CAMs9,10 and other problems seen predominantly overseas, such as contamination, adulteration, substitution, variable dosage, dubious quality control and inappropriate labelling.10,11 Doctors need to directly ask about the use of CAMs in a non-judgmental manner to better ascertain the risks of drug interactions and potential side effects from CAMs.
Giving children medicines that have not been tested in children is another important quality use of medicines concern. In households we surveyed where there were children under the age of 15 years, a third of these children had been administered CAMs. Other surveys have reported use of CAM therapies during pregnancy and in our last survey only 36.2% of respondents thought that CAMs were not safe during pregnancy.3 Although few CAMs have been shown to be teratogenic, most have not been sufficiently tested to rule out adverse fetal effects and CAMs should not be used during pregnancy.
Users of CAMs had a lower quality of life in all of the physical and psychological domains of the SF36 questionnaire except physical functioning. However, these results should be interpreted with caution as this was a cross-sectional study and longitudinal quality-of-life scores are not available from before self-medication with CAMs began. In some cases the public, particularly older respondents, are using CAMs because of chronic health problems and thus they may have had low quality-of-life scores before starting medication. Also, surveys are open to recall bias and potential bias if non-respondents differ from the respondents. However, response rates were high in all three of our surveys and on retesting 10% of respondents the answers remained consistent.
It has been argued that the rising use of CAMs does not change the rules for informed consent.12 If a physician or CAM therapist advocates or prescribes CAMs then it is their legal duty to warn the patient of:
the material hazards,
possible complications that could occur,
reasonable alternatives, and
the effects of non-treatment.
Prescribers must also have a thorough knowledge of the patient’s medical history, pertinent family history, current medications and risk of pregnancy. This responsibility is incumbent even on those who advise on CAMs at the point of sale; however, most CAMs are not sold with general product information to alert the consumer to possible risks. Further, the scientific evidence that underpins the role of CAMs for the broad range of conditions for which they are used is far from complete. The quality of the evidence for specific CAM medicines or interventions for specific medical conditions ranges from first class to nonexistent.13-17 Even in areas where a substantive amount of work has been undertaken, the research suffers from a lack of methodological rigour.
The Chairman of the Australian Health Ethics Committee, National Health and Medical Research Council, has argued the ethics of promoting products or therapies that may only have a placebo effect.18 Those who practise or recommend CAM have ethical responsibilities to inform patients when the therapy is unproven as well as of any potential risks. Patients offered CAM interventions must not be denied access to standard proven therapies. Equally, as the evidence base for CAM increases, any CAM medicine or intervention with appropriate evidence should be seen as a reasonable alternative to a conventional medical approach.
Our results clearly showed that half the population thought that CAMs were independently tested by the TGA before being allowed to be sold. Currently, the TGA has limited capacity (as the nature of TGA funding requires full cost recovery) and audits less than 1% of CAMs on the market. Most CAMs are “Listed” (L classification) by the TGA on the Australian Register of Therapeutic Goods (their stated contents and safe manufacture have been accepted without audit and without proof of efficacy) and can be sold and advertised with “low level” claims. In contrast, “Registered” (R classification) medicines have been assessed for quality, safety and efficacy.
The Expert Committee on Complementary Medicines in the Australian Health System, set up by the Australian Government after the Pan Pharmaceutical crisis, recommended an increase in the random and targeted assessment of the indications and claims held by sponsors of CAMs.19 The committee made 49 major recommendations for improved regulation of complementary medicines in Australia. In 2005, the Australian Government accepted all but one of these recommendations and is now implementing the process. These changes will strengthen the regulatory framework for CAMs and CAM therapists in Australia.
1 CAM users by demographic variables
|
n |
% (95% CI) |
|||||||||||||
Age (years) |
|
|
|||||||||||||
15–24 |
263 |
53.0% (48.3%–57.7%) |
|||||||||||||
25–34 |
296 |
59.9% (55.2%–64.5%) |
|||||||||||||
35–44 |
318 |
57.5% (53.0%–61.9%) |
|||||||||||||
45–54 |
284 |
54.5% (49.9%–59.0%) |
|||||||||||||
55–64 |
209 |
52.4% (47.1%–57.6%) |
|||||||||||||
≥ 65 |
204 |
37.0% (32.8%–41.4%) |
|||||||||||||
Sex |
|
|
|||||||||||||
Male |
679 |
45.9% (43.2%–48.6%) |
|||||||||||||
Female |
896 |
58.4% (55.7%–60.9%) |
|||||||||||||
Country of birth |
|||||||||||||||
Australia |
1202 |
53.6% (51.4%–55.8%) |
|||||||||||||
UK and Ireland |
182 |
49.6% (44.1%–55.1%) |
|||||||||||||
Europe (minus UK/Ireland) |
94 |
47.7% (40.0%–55.0%) |
|||||||||||||
Asian country |
46 |
45.1% (34.9%–55.8%) |
|||||||||||||
Other |
50 |
48.1% (37.8%–58.5%) |
|||||||||||||
Marital status |
|
|
|||||||||||||
Married/de facto |
997 |
53.5% (51.0%–55.9%) |
|||||||||||||
Never married |
142 |
55.3% (48.6%–61.7%) |
|||||||||||||
Separated/divorced |
60 |
35.9% (28.2%–43.9%) |
|||||||||||||
Widowed |
375 |
51.7% (47.8%–55.6%) |
|||||||||||||
Post-secondary school education |
|||||||||||||||
No |
707 |
46.1% (43.5%–48.7%) |
|||||||||||||
Yes |
868 |
58.6% (55.9%–61.3%) |
|||||||||||||
Household income (AUD$) |
|||||||||||||||
≤ $30 000 |
375 |
40.5% (37.2%–43.9%) |
|||||||||||||
$30 001–80 000 |
676 |
58.5% (55.5%–61.5%) |
|||||||||||||
> $80 000 |
367 |
60.9% (56.5%–64.8%) |
|||||||||||||
Not stated |
156 |
47.3% (41.7%–52.7%) |
|||||||||||||
Area |
|
|
|||||||||||||
Metro |
1145 |
54.2% (51.9%–56.4%) |
|||||||||||||
Country |
429 |
47.6% (44.1%–51.0%) |
|||||||||||||
OVERALL |
1574 |
52.2% (50.3%–54.1%) |
|||||||||||||
Statistically significant differences between CAM users and non-users are highlighted (P < 0.05). |
|||||||||||||||
2 Longitudinal comparison of the use of CAMs
|
|
1993 |
2000 |
2004 |
|||||||||||
Vitamins* |
Total |
37.6% |
36.4% |
39.2% |
|||||||||||
Males |
33.8% |
31.5% |
35.6% |
||||||||||||
Females |
41.2% |
41.2% |
42.7% |
||||||||||||
Herbal medicines |
Total |
9.9% |
13.4% |
20.6% |
|||||||||||
Males |
8.6% |
10.3% |
16.1% |
||||||||||||
Females |
11.1% |
16.6% |
24.9% |
||||||||||||
Mineral supplements |
Total |
9.2% |
10.6% |
13.6% |
|||||||||||
Males |
8.1% |
9.6% |
11.2% |
||||||||||||
Females |
10.3% |
11.5% |
16.0% |
||||||||||||
Aromatherapy oils |
Total |
3.5% |
15.3% |
11.2% |
|||||||||||
Males |
1.9% |
8.2% |
5.4% |
||||||||||||
Females |
5.2% |
22.2% |
16.7% |
||||||||||||
Soy products |
Total |
— |
— |
3.8% |
|||||||||||
Males |
— |
— |
2.3% |
||||||||||||
Females |
— |
— |
5.2% |
||||||||||||
Chinese medicines |
Total |
1.8% |
3.2% |
2.3% |
|||||||||||
Males |
1.6% |
2.6% |
2.0% |
||||||||||||
Females |
2.1% |
3.7% |
2.7% |
||||||||||||
Homeopathic medicines |
Total |
4.4% |
4.3% |
2.2% |
|||||||||||
Males |
3.2% |
3.2% |
1.5% |
||||||||||||
Females |
5.5% |
5.2% |
2.9% |
||||||||||||
Other |
Total |
15.8% |
11.3% |
6.1% |
|||||||||||
Males |
9.9% |
9.3% |
5.5% |
||||||||||||
Females |
21.5% |
13.3% |
6.7% |
||||||||||||
Total CAM users (at least one product) |
Total |
48.5% |
52.1% |
52.2% |
|||||||||||
Males |
42.0% |
43.9% |
45.9% |
||||||||||||
Females |
54.8% |
60.0% |
58.4% |
||||||||||||
* Not calcium, iron or vitamins prescribed by a doctor. Highlighted rows are significant at P < 0.05 by χ2 test. |
|||||||||||||||
3 Reasons given for using CAMs
|
Male |
Female |
All |
||||||||||||
General health |
68.7% |
71.1% |
70.1% |
||||||||||||
Muscles, bones or joints |
21.6% |
20.3% |
20.9% |
||||||||||||
Immune system |
18.9% |
17.7% |
18.2% |
||||||||||||
Nerves or stress |
8.8% |
16.2% |
13.0% |
||||||||||||
Blood or circulation |
8.9% |
10.0% |
9.5% |
||||||||||||
PMS/menopause |
— |
14.8% |
8.4% |
||||||||||||
Skin |
6.0% |
9.6% |
8.1% |
||||||||||||
Lung or sinuses |
6.4% |
5.5% |
5.9% |
||||||||||||
Stomach or bowel |
3.2% |
6.9% |
5.3% |
||||||||||||
Bladder or kidneys |
1.5% |
3.2% |
2.5% |
||||||||||||
Prostate |
2.9% |
— |
1.3% |
||||||||||||
Other |
10.3% |
12.1% |
11.3% |
||||||||||||
Don’t know |
1.6% |
0.6% |
1.0% |
||||||||||||
Multiple responses allowed. Significant differences (P < 0.05) between sexes are highlighted. |
|||||||||||||||
5 Use of CAM therapists in 1993, 2000 and 2004
|
1993 |
2000 |
2004 |
||||||||||||
|
Total |
Total |
Male |
Female |
Total |
||||||||||
Chiropractor |
15.0% |
16.7% |
16.1% |
17.3% |
16.7% |
||||||||||
Naturopath/Natural Therapist |
5.0% |
6.0% |
3.6% |
7.6% |
5.7% |
||||||||||
Acupuncturist |
2.0% |
2.8% |
1.2% |
3.1% |
2.1% |
||||||||||
Homeopath |
1.2% |
1.2% |
0.1% |
0.9% |
0.5% |
||||||||||
Iridologist |
0.8% |
1.2% |
0.2% |
1.4% |
0.8% |
||||||||||
Reflexologist |
0.7% |
1.2% |
1.2% |
0.8% |
1.0% |
||||||||||
Aromatherapist |
0.6% |
1.3% |
0.6% |
1.5% |
1.1% |
||||||||||
Herbal therapist |
0.4% |
0.9% |
1.0% |
1.5% |
1.9% |
||||||||||
Osteopath |
0.2% |
0.4% |
0.4% |
0.4% |
0.4% |
||||||||||
Other |
1.8% |
1.2% |
3.9% |
5.6% |
4.8% |
||||||||||
Total (any visited) |
20.3% |
23.3% |
23.6% |
29.3% |
26.5% |
||||||||||
- Alastair H MacLennan1
- Stephen P Myers2
- Anne W Taylor3
- 1 Department of Obstetrics and Gynaecology, University of Adelaide, North Adelaide, SA.
- 2 Australian Centre for Complementary Medicine Education and Research, Southern Cross University, Lismore, NSW.
- 3 Population Research and Outcomes Unit, South Australian Department of Health, Adelaide, SA.
Alastair MacLennan and Stephen Myers were members of the Australian Government’s Expert Committee on Complementary Medicines in the Australian Health System.
- 1. Barnes P, Powell-Griner E, McFann K, Nahin R. Complementary and alternative medicine among adults: United States, 2002. Adv Data 2004; (343): 1-19.
- 2. MacLennan AH, Wilson DH, Taylor AW. Prevalence and cost of alternative medicine in Australia. Lancet 1996; 347: 569-573.
- 3. MacLennan AH, Wilson DH, Taylor AW. The escalating cost and prevalence of alternative medicine. Prev Med 2002; 35: 166-173.
- 4. Wilson D, Wakefield M, Taylor A. The South Australian Health Omnibus Survey. Health Prom J Aust 1992; 2: 47-49.
- 5. Amato P, Marcus DM. Review of alternative therapies for the treatment of menopausal symptoms. Climacteric 2003; 6: 278-284.
- 6. MacLennan AH, Taylor AW, Wilson DH. Hormone therapy use after the Women’s Health Initiative. Climacteric 2004; 7: 138-142.
- 7. Huntley AL, Ernst E. A systematic review of herbal medicinal products for the treatment of menopausal symptoms. Menopause 2003; 10: 465-476.
- 8. D’Arcy PF. Adverse reactions and interactions with herbal medicines. Part 2: drug interactions. Adverse Drug React Toxicol Rev 1993; 12: 147-162.
- 9. Ernst E. Harmless herbs? Am J Med 1998; 104: 170-178.
- 10. Drew AK, Myers SP. Safety issues in herbal medicines: implications for the health professions. Med J Aust 1997; 166: 538-541. <MJA full text>
- 11. Cui J, Garle M, Eheroth P, Bjorkhem I. What do commercial ginseng preparations contain? Lancet 1994; 344: 134.
- 12. Mayes G. Does the rising use of complementary and alternative medicine change the rules for informed consent? Medscape Ob/Gyn Womens Health 2004; 9(1). Available at: http://www.medscape.com/viewarticle/481399 (accessed Oct 2005).
- 13. Ernst E. Herbal medicinal products: an overview of systematic reviews and meta-analyses. Perfusion 2001; 16: 398-404.
- 14. Melchart D, Linde K, Fischer P, Kaesmayr J. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev 2000; (2): CD000530.
- 15. Jepson RG, Kleijnens J, Leng GC. Garlic for peripheral arterial occlusive disease. Cochrane Database Syst Rev 2000; (2): CD000095.
- 16. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2005; (2): CD002946.
- 17. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause 2004; 11: 11-33.
- 18. Breen KJ. Ethical issues in the use of complementary medicines. Climacteric 2003; 6: 268-272.
- 19. Expert Committee on Complementary Medicines in the Australian Health System. Complementary medicines in the Australian health system. Report to the Parliamentary Secretary to the Minister for Health and Ageing. Canberra: Commonwealth of Australia, 2003. Available at: http://www.tga.gov.au/docs/html/cmreport1.htm (accessed Oct 2005).





Abstract
Objective: To survey the use, cost, beliefs and quality of life of users of complementary and alternative medicine (CAM).
Design: A representative population survey conducted in 2004 with longitudinal comparison to similar 1993 and 2000 surveys.
Participants: 3015 South Australian respondents over the age of 15 years (71.7% participation).
Results: In 2004, CAMs were used by 52.2% of the population. Greatest use was in women aged 25–34 years, with higher income and education levels. CAM therapists had been visited by 26.5% of the population. In those with children, 29.9% administered CAMs to them and 17.5% of the children had visited CAM therapists. The total extrapolated cost in Australia of CAMs and CAM therapists in 2004 was AUD$1.8 billion, which was a decrease from AUD$2.3 billion in 2000. CAMs were used mostly to maintain general health. The users of CAM had lower quality-of-life scores than non-users. Among CAM users, 49.7% used conventional medicines on the same day and 57.2% did not report the use of CAMs to their doctor. About half of the respondents assumed that CAMs were independently tested by a government agency; of these, 74.8% believed they were tested for quality and safety, 21.8% for what they claimed, and 17.9% for efficacy.
Conclusions: Australians continue to use high levels of CAMs and CAM therapists. The public is often unaware that CAMs are not tested by the Therapeutic Goods Administration for efficacy or safety.