The emergence of pandemic (H1N1) 2009 influenza posed challenges to the Australian health system.1,2 Soon after the first locally acquired cases were recognised in Australia in May 2009, it became clear that, despite the presence of pandemic plans and a variety of surveillance systems for influenza,3 there was a gap in surveillance at the severe end of the clinical spectrum.
Data on severity are important because an increase in severe respiratory complications such as pneumonia in adults could signal a change in the virulence of pandemic viral strains. Although some newly emerged influenza strains, such as H5N1, have been associated with direct viral-associated lung disease,4 it has been suggested that mortality in previous pandemics was associated with secondary bacterial pneumonia,5,6 emphasising the importance of surveillance for pneumonia as well as influenza during a pandemic. This gap in surveillance was common in many countries, and a variety of systems were rapidly set up or augmented during 2009 to gather information on hospitalisations.1,7-12
In Australia, existing systems were rapidly scaled up to meet the challenge, while state and territory health departments provided data from hospitals to the Australian Government Department of Health and Ageing.13 Other sources of influenza surveillance data were limited to patients in intensive care units (ICUs) or hospitalised children.14,15 A variety of systems were set up in jurisdictional health departments, but these relied on personal networks of public health physicians, laboratory data and automated reporting systems.13,16
Thus, at the onset of the H1N1 pandemic in 2009, there were no reliable, comprehensive, consistent and rapidly available data on adult acute respiratory hospitalisations outside the ICU setting in Australia. The Influenza Complications Alert Network (FluCAN) was designed to fill this gap by providing data from sentinel hospitals on acute respiratory hospitalisations, including ICU admissions. Here, we present its initial findings for the first 6 months, and compare FluCAN data on pandemic (H1N1) 2009 influenza with publicly available data from the national surveillance system13 and published data from the Australian and New Zealand Intensive Care Society (ANZICS) surveillance system.14,17
Rapid collection and reporting of data on adult patients with laboratory-confirmed influenza hospitalised at 10 sentinel sites (nine in Australia and one in New Zealand);
Detailed data collection on adult patients admitted to the same hospitals with laboratory-confirmed influenza or community-acquired pneumonia (with or without influenza).
Standard case definitions were used for community-acquired pneumonia,18 influenza and comorbidities. Influenza testing was initiated by local clinicians based on clinical indications and was not funded by FluCAN. All influenza cases were confirmed using real-time reverse transcriptase polymerase chain reaction (PCR) assays using standard primers. All tests were performed in local or referral laboratories accredited by the National Association of Testing Authorities. Patients with community-acquired pneumonia were included if they exhibited clinical features suggestive of lower respiratory tract infection, had a chest x-ray demonstrating a new infiltrate consistent with pneumonia, and had onset in a community setting. Comorbidities were recorded if they were documented in the clinical notes, medical sources or via self-report by the patient.
National notifications of hospital admissions, ICU admissions and deaths related to pandemic (H1N1) 2009 influenza were obtained from the Australian Influenza Surveillance Report.13 In addition, data on ICU admissions were obtained from publications arising from the ANZICS surveillance system.14,17 Hospital bed capacities were derived from local sources and official hospital statistics for use as denominator data.19
The eight public acute care hospitals in six Australian jurisdictions reporting FluCAN data in 2009 (Box 1) represented 10% of all Australian hospitals with over 200 beds (n = 79). The 3461 beds in the sentinel hospitals represent 6.4% of national bed capacity (n = 54 338).19 All but one hospital was in a major urban area. From 1 July to 4 December 2009, 190 hospital-weeks of observation were undertaken (136 [71.6%] prospectively).
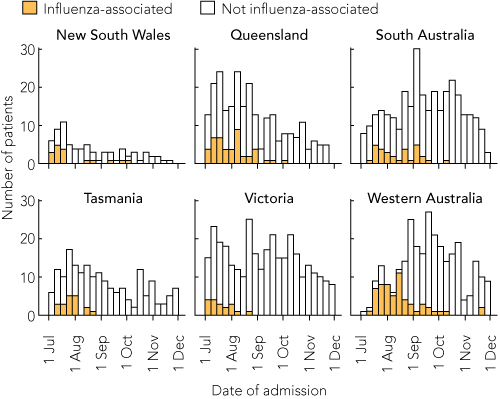
A total of 538 patients were recorded as being hospitalised with PCR-confirmed influenza; of these cases, 465 (86.4%) were due to pandemic (H1N1) 2009 influenza (representing 9.3% of the 4992 admissions for the pandemic strain notified nationally), with the remainder due to seasonal strains of influenza A. There were also 1468 patients hospitalised with radiologically confirmed community-acquired pneumonia, 718 (48.9%) of whom were tested for influenza; 194 cases (13.2%) were found to be associated with influenza, mostly the pandemic strain (163, 11.1%) (Box 1).
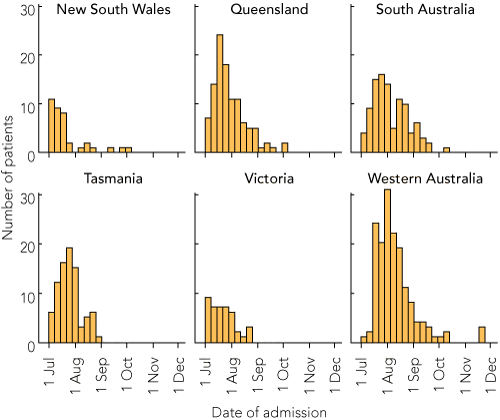
There was marked geographic variation in the distribution of the 538 influenza cases, with lower than expected caseloads in Victoria (which had 25.1% of total beds in the sentinel hospitals, but only 8.7% of the total influenza cases) and New South Wales (13.0% of beds and 6.9% of influenza cases), and higher than expected caseloads in the other states, especially Western Australia (16.7% of beds and 29.6% of influenza cases) (Box 1). This was mainly due to the timing of data collection, which commenced after the peak of cases in Victoria in mid to late June 200920 but before the majority of cases in WA (Box 2). This timing issue was also evident in the proportion of pandemic (H1N1) 2009 influenza cases in each state, which was as low as 64.9% in NSW and as high as 97.0% in South Australia (Box 1).
The epidemiology of pneumonia was less affected by the timing of data collection, except for higher than expected numbers of admissions in Tasmania and lower than expected numbers of admissions in NSW (Box 1 and Box 3). Compared with influenza patients, a similar proportion of patients with pneumonia were men, but patients were older, with a median age of 69 years (interquartile range, 18–101 years). There were 107 Indigenous patients admitted with pneumonia (7.3%).
Box 4 compares pandemic (H1N1) 2009 influenza cases recorded in FluCAN with national notifications13 and intensive care admissions.14,17 As a proportion of nationally notified hospitalisations, the number of FluCAN-reported cases was higher than expected (465/4992, 9.3%), based on the number of beds in the sentinel hospitals and the limited period of observation. FluCAN reported 102 ICU admissions (15.0% of the nationally reported number), again more than expected given the proportion of national ICU bed capacity in the sentinel hospitals.
Compared with national notifications, FluCAN-reported hospitalised patients had a higher median age, a similar proportion of women, and a higher proportion of pregnant women (Box 4). Most pregnant women (29, 72.5%) were in their third trimester. Of note, 40% of pregnant women (16/40) admitted to FluCAN hospitals with pandemic (H1N1) 2009 influenza were over the age of 45 years; these women are not included in the national notifications of women of childbearing age (age range, 15–44 years). FluCAN reported a lower proportion of admitted Indigenous patients but a higher ascertainment rate for Indigenous status (96.8% v 80.7%), and a higher rate of at least one comorbidity. Prominent comorbidities included asthma, obesity and immunosuppression. Almost 30% of admitted patients for whom information was available were current smokers. Median length of stay and death rate were slightly higher in FluCAN, although the latter can be partly explained by FluCAN’s inclusion of data on post-discharge deaths.
Compared with the national notifications, FluCAN reported a higher proportion of patients with pandemic (H1N1) 2009 influenza admitted to ICUs (Box 4). For ICU patients, demographic and risk factor data were generally similar between patients reported in FluCAN and both the national notifications and ANZICS data — median age, proportion of women, and the proportion of patients with at least one comorbidity were very similar. FluCAN reported a higher proportion of pregnant women admitted to ICUs than both other systems. Specific comorbidities available from ANZICS data were very similar to those reported by FluCAN, as were the proportion of patients with pneumonia, length of stay and death rate. This level of detail was not available from the national notification data.
Initial information from Australia and overseas on the severity of pandemic (H1N1) 2009 influenza infection suggested that the illness was relatively mild and mainly affected children and young adults. Pregnancy, obesity, asthma, chronic obstructive pulmonary disease and some other non-respiratory comorbidities appeared to be associated with more severe disease in a minority of patients.21-23 Subsequent reports have shown a range of estimates for underlying comorbidities and particular populations at greater risk of severe disease. A recent report reviewed hospitalisation data globally,8 and there have been Australian reports of hospitalisations in single hospitals in Sydney24 and Darwin,25 or clusters of hospitals in specific geographic regions.16,26 FluCAN data are broadly in agreement with the estimates of frequency of vulnerable groups, proportion admitted to ICUs, and mortality in these other studies. Only FluCAN has thus far included estimates of post-discharge health service use and deaths.
Data on pneumonia admissions in Australia are provided from routinely collected sources that are not timely and generally lack specificity in diagnosis and details of comorbidities. The Australian Community-Acquired Pneumonia Study (ACAPS) reported more detailed information on pneumonia from six hospitals (including two that contributed to FluCAN) in three states.18 Compared with patients with pneumonia in FluCAN, ACAPS patients were similar in age and the proportion of female patients was similar. There were large differences in Indigenous status and underlying comorbidities, perhaps because almost half of all ACAPS patients were admitted to a single tertiary referral hospital in Melbourne.18
There were several limitations to the FluCAN surveillance system. For a variety of reasons, influenza PCR tests were not done routinely for all patients with respiratory illnesses in some hospitals, despite national guidelines encouraging testing for hospitalised and high-risk patients with influenza-like illness.27 Thus, an unknown number of admissions during this period may have been due to influenza but not diagnosed and therefore not included in the data presented here. In its first year, FluCAN attempted to collect as much relevant information as possible, but there was a surprising lack of consistent recording of important variables such as smoking, vaccination status, weight and height. Ongoing surveillance will provide a comparison during a non-pandemic season and hopefully address some of the limitations of data collection in 2009.1,8,14
1 Patients admitted to Australian FluCAN hospitals with influenza and pneumonia, July to December 2009
4 Hospitalisations and intensive care unit (ICU) admissions for pandemic (H1N1) 2009 influenza in Australia in 2009: a comparison of FluCAN data, nationally notified cases13 and ICU admissions reported by ANZICS1,7*
Received 15 August 2010, accepted 7 November 2010
- Paul M Kelly1
- Tom Kotsimbos2
- Anna Reynolds1
- Richard Wood-Baker3,4
- Bob Hancox5,6
- Simon G A Brown7
- Mark Holmes8,9
- Graham Simpson10,11
- Simon Bowler12,13
- Grant Waterer14,15
- Louis B Irving16,17
- Christine Jenkins18,19
- Phillip J Thompson20
- Allen C Cheng21,22
- 1 National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT.
- 2 Department of Allergy, Immunology and Respiratory Medicine, The Alfred Hospital and Monash University, Melbourne, VIC.
- 3 Department of Respiratory Medicine, Royal Hobart Hospital, Hobart, TAS.
- 4 Respiratory Research Group, Menzies Research Institute, Hobart, TAS.
- 5 Department of Respiratory Medicine, Waikato Hospital, Hamilton, New Zealand.
- 6 Department of Preventive and Social Medicine, University of Otago, Dunedin, New Zealand.
- 7 Centre for Clinical Research in Emergency Medicine, Royal Perth Hospital, Western Australian Institute for Medical Research, and the University of Western Australia, Perth, WA.
- 8 University of Adelaide, Adelaide, SA.
- 9 South Australia Lung Transplant Services, Royal Adelaide Hospital, Adelaide, SA.
- 10 Thoracic Medicine Department, Cairns Base Hospital, Cairns, QLD.
- 11 James Cook University, Cairns, QLD.
- 12 Department of Respiratory Medicine, Mater Adult Hospital, Brisbane, QLD.
- 13 University of Queensland, Brisbane, QLD.
- 14 Royal Perth Hospital, Perth, WA.
- 15 School of Medicine and Pharmacology, University of Western Australia, Perth, WA.
- 16 Department of Respiratory and Sleep Medicine, Royal Melbourne Hospital, Melbourne, VIC.
- 17 Department of Medicine, University of Melbourne, Melbourne, VIC.
- 18 Department of Respiratory Medicine, Concord Hospital, Sydney, NSW.
- 19 University of Sydney and Woolcock Institute of Medical Research, Sydney, NSW.
- 20 Lung Institute of Western Australia and the Centre for Asthma, Allergy and Respiratory Research, University of Western Australia, Perth, WA.
- 21 Infectious Diseases Unit, The Alfred Hospital, Melbourne, VIC.
- 22 Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC.
We gratefully acknowledge the hard work and professionalism of the FluCAN senior coordinators, Janine Roney and Anne Lickliter at The Alfred, and the local coordinators and data collectors at each hospital: Chris Tuffery (Waikato); Susan Wagg (Royal Hobart); Kirsty Herewane, Louise Milazzo, Jenny McGrath and Mary-Jo Neale (Royal Adelaide); Ellen MacDonald and Jenny Chamberlain (Royal Perth); Carolyn Fennell and Kerrie Wade (Concord); Michelle Thompson and Gerri Shandler (Royal Melbourne); Megan Martin and Fiona MacPhee (The Mater); Sue Richmond and Ann Carroll (Cairns). Thanks to Ingrid Van Rensberg for assistance with data collation, analysis and secretariat work, and Ivan Hanigan for development of the database. We acknowledge the assistance of David Begg (Thoracic Society of Australia and New Zealand secretariat) in organising teleconferences and distribution of weekly reports. This project was funded by a National Health and Medical Research Council (NHMRC) Strategic Grant. Allen Cheng was supported by an NHMRC Health Professional Research Training Fellowship and Paul Kelly by a Career Development Award. The Master of Applied Epidemiology program is funded by the Australian Government Department of Health and Ageing.
Allen Cheng is an investigator on a surveillance study of the safety of influenza vaccine funded by CSL Ltd. The Thoracic Society of Australia and New Zealand provided support for Paul Kelly to travel to its annual scientific conference to present findings related to this paper. Bob Hancox is a board member of the Asthma Foundation (New Zealand) and has received royalties from McGraw-Hill for a book on lung function. Richard Wood-Baker has conducted research sponsored by GlaxoSmithKline (GSK) and Gilead and had travel expenses paid by Boehringer Ingelheim for a presentation at Airways 2008. Louis Irving has received payment for lectures and development of educational presentations from GSK and AstraZeneca, and has had travel expenses paid by AstraZeneca for attendance at a European Respiratory Society conference. Mark Holmes is a Novartis board member, for which he receives payment and reimbursement of travel expenses, and he has received payment for lectures from Medimark International, AstraZeneca, Pfizer and Boehringer Ingelheim.
- 1. Kotsimbos T, Waterer G, Jenkins C, et al. Influenza A/H1N1_09: Australia and New Zealand’s winter of discontent. Am J Respir Crit Care Med 2010; 181: 300-306.
- 2. Bishop JF, Murnane MP, Owen R. Australia’s winter with the 2009 pandemic influenza A (H1N1) virus. N Engl J Med 2009; 361: 2591-2594.
- 3. Kaczmarek M, Owen R, Barr IG. Annual report of the National Influenza Surveillance Scheme, 2008. Commun Dis Intell 2010; 34: 8-22.
- 4. de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12: 1203-1207.
- 5. Brundage JF, Shanks GD. What really happened during the 1918 influenza pandemic? The importance of bacterial secondary infections [letter]. J Infect Dis 2007; 196: 1717-1718; author reply 1718-1719.
- 6. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198: 962-970.
- 7. Jain R, Goldman RD. Novel influenza A(H1N1): clinical presentation, diagnosis, and management. Pediatr Emerg Care 2009; 25: 791-796.
- 8. Bautista E, Chotpitayasunondh T, Gao Z, et al; Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362: 1708-1719.
- 9. Donaldson LJ, Rutter PD, Ellis BM, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ 2009; 339: b5213.
- 10. Louie JK, Acosta M, Jamieson DJ, Honein MA; California Pandemic (H1N1) Working Group. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med 2010; 362: 27-35.
- 11. Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302: 1872-1879.
- 12. Centers for Disease Control and Prevention. Deaths and hospitalizations related to 2009 pandemic influenza A (H1N1) — Greece, May 2009–February 2010. MMWR Morb Mortal Wkly Rep 2010; 59: 682-686.
- 13. Australian Influenza Surveillance Report. No. 25, 2010, reporting period: 19 June 2010 – 25 June 2010. Canberra: Australian Government Department of Health and Ageing, 2010. http://www.healthemergency.gov.au/internet/healthemergency/publishing.nsf/Content/ozflu2010-apr-jun-pdf-cnt.htm/$File/ozflu-no25-2010.pdf (accessed Jan 2011).
- 14. ANZIC Influenza Investigators and Australasian Maternity Outcomes Surveillance System. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ 2010; 340: c1279.
- 15. Zurynski YA, Lester-Smith D, Festa MS, et al. Enhanced surveillance for serious complications of influenza in children: role of the Australian Paediatric Surveillance Unit. Commun Dis Intell 2008; 32: 71-76.
- 16. Cretikos MA, Muscatello DJ, Patterson J, et al; New South Wales public health network. Progression and impact of the first winter wave of the 2009 pandemic H1N1 influenza in New South Wales, Australia. Euro Surveill 2009; 14 (42): pii=19365.
- 17. Webb SA, Pettila V, Seppelt I, et al; ANZIC Influenza Investigators. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 2009; 361: 1925-1934.
- 18. Charles PG, Whitby M, Fuller AJ, et al. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis 2008; 46: 1513-1521.
- 19. Australian Institute of Health and Welfare. Australian hospital statistics 2008–09. Canberra: AIHW, 2010. (AIHW Cat. No. HSE 84.)
- 20. Victorian Infectious Diseases Reference Laboratory. The 2009 Victorian influenza vaccine effectiveness audit report. Report no. 29, week ending 15/11/2009. Melbourne: VIDRL, 2009. http://www.vidrl.org.au/surveillance/flu%20reports/flurpt09/pdf_files/flu0929.pdf (accessed Jan 2011).
- 21. Dawood FS, Jain S, Finelli L, et al; Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360: 2605-2615.
- 22. Centers for Disease Control and Prevention. Hospitalized patients with novel influenza A (H1N1) virus infection — California, April–May, 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 536-541.
- 23. Health Protection Agency, Health Protection Scotland, National Public Health Service for Wales, HPA Northern Ireland Swine influenza investigation teams. Epidemiology of new influenza A (H1N1) virus infection, United Kingdom, April – June 2009. Euro Surveill 2009; 14 (22): pii=19232.
- 24. Chang YS, van Hal SJ, Spencer PM, et al. Comparison of adult patients hospitalised with pandemic (H1N1) 2009 influenza and seasonal influenza during the “PROTECT” phase of the pandemic response. Med J Aust 2010; 192: 90-93. <MJA full text>
- 25. Flint S, Su J-Y, Scott L, Krause V. The early experience of pandemic (H1N1) 2009 influenza in the Northern Territory, Australia. NT Dis Control Bull 2009; 16 (2): 1-8.
- 26. Denholm JT, Gordon CL, Johnson PD, et al. Hospitalised adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. Med J Aust 2010; 192: 84-86.
- 27. Australian Government Department of Health and Ageing. Protect Phase: Annex to the Australian Health Management Plan for Pandemic Influenza. Version 3, 21 September 2009. Canberra: Australian Government, 2009.





Abstract
Objective: To describe the epidemiology of adult patients hospitalised with influenza or pneumonia during a pandemic season in a sentinel network in Australia.
Design, participants and setting: Prospective case series of adult hospital admissions to eight acute care general public hospitals (Influenza Complications Alert Network [Flu CAN] sentinel hospitals) in six Australian jurisdictions, 1 July to 4 December 2009.
Main outcome measures: Demographic, clinical and outcome measures in patients admitted with laboratory-confirmed pandemic (H1N1) 2009 influenza in the sentinel hospitals compared with data from national notifications and intensive care unit (ICU) surveillance; admissions for influenza and pneumonia over time in each jurisdiction.
Results: During 190 hospital-weeks of observation, there were 538 influenza admissions. Of these, 465 patients (86.4%) had the pandemic strain, representing 9.3% of total admissions with pandemic (H1N1) 2009 influenza (n = 4992) recorded nationally in 2009. Of these patients, 250/465 (53.8%) were women, 67/453 (14.8%) were Indigenous, and the median age was 46 years (interquartile range, 29–58 years). Comorbidities were present in 354/464 patients (76.3%), and 40 were pregnant (30.3% of women aged 15–49 years). FluCAN reported that 102 patients (21.9%) were admitted to ICUs, and of patients admitted to hospital, 26 (5.6%) died. FluCAN results were very similar to national notification data and published ICU admissions data. Of those who were followed to 30 days after discharge, 30 (6.5%) were readmitted. Of 1468 patients hospitalised with pneumonia, 718 (48.9%) were tested for influenza and 163 (11.1%) were co-infected with the pandemic strain.
Conclusions: Sentinel surveillance systems can provide important and reliable information in a timely fashion and can monitor changes in severity of influenza during a pandemic season.