In early April 2009, a novel influenza A virus was identified in Mexico and Southern California.1 The subsequent rapid international spread and sustained community transmission in the Americas, Europe, New Zealand and Australia resulted in the World Health Organization escalating the pandemic influenza response to Phase 6 on 11 June 2009.2
The Australian Health Management Plan for Pandemic Influenza, which includes the ALERT, DELAY, CONTAIN, SUSTAIN, CONTROL and RECOVER phases of response,3 was predicated on pandemic influenza causing high morbidity and mortality. However, pandemic (H1N1) 2009 influenza appeared to be causing milder disease. Cases of severe disease and deaths have been reported,4 in particular, pregnant women could have severe disease.5,6 A modified strategy, the “PROTECT” phase, was thus enacted on 17 June 2009.7 It focused on identifying and treating infection in people with moderate to severe disease and those with certain risk factors (pregnancy and underlying chronic diseases), controlling outbreaks in institutions, and monitoring hospitalisation.8
The PROTECT phase coincided with the start of the influenza season in Sydney, and the surge in hospital admissions of patients with pandemic (H1N1) 2009 influenza coincided with the expected surge in seasonal influenza. This situation allowed us to compare patient characteristics, clinical features and outcomes of infection with pandemic (H1N1) 2009 influenza and seasonal influenza. Part of this work was presented at the 14th Congress of the Asian Pacific Society of Respirology in Seoul, Korea, 14–18 November 2009 (Poster no. APSR 2009-594).
Health-care-associated influenza was defined as onset of symptoms more than 4 days after admission, to correspond with the upper limit of influenza virus incubation.9
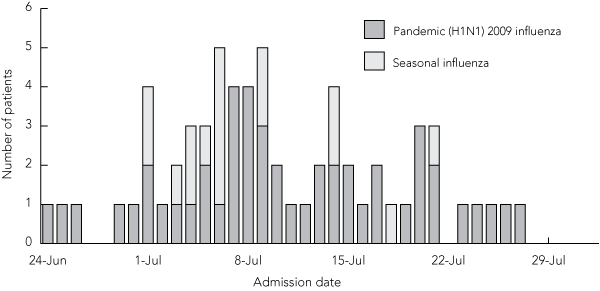
Sixty-five patients were admitted to Liverpool Hospital with a laboratory-confirmed diagnosis of influenza between 17 June and 31 July 2009. One of these patients had been admitted for ischaemic gut, and was excluded from the analysis because the patient did not have a concurrent influenza-like illness. Of the remaining 64 patients, 48 tested positive for pandemic (H1N1) 2009 influenza and the remaining 16 tested positive for seasonal influenza A (11 for subtype H3 and five for untypeable non-pandemic [H1N1] 2009 influenza). The epidemic curve showed that the peak of the outbreak occurred around 8 July (Box 1). Five of the 64 cases of influenza (all pandemic [H1N1] 2009) were health-care associated.
Box 2 shows demographic characteristics and presenting features and outcomes for patients admitted with pandemic (H1N1) 2009 and seasonal influenza. Patients with pandemic (H1N1) 2009 influenza were significantly younger than those presenting with seasonal influenza (mean age, 45 years v 64 years; P < 0.01). Patients with seasonal influenza were more commonly immunosuppressed than patients with pandemic (H1N1) 2009 (P < 0.05). Although a relationship with pregnancy (patients in their postpartum period or pregnant) was more common in the pandemic (H1N1) 2009 group (eight patients v one patient with seasonal influenza), this difference did not reach statistical significance (Box 2).
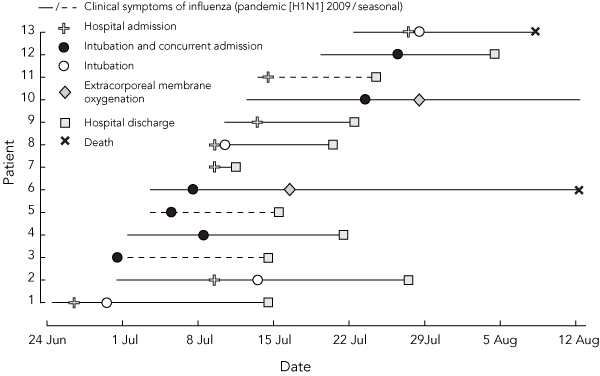
Thirteen patients were admitted to the intensive care unit (ICU) — three with seasonal and 10 with pandemic (H1N1) 2009 influenza. The clinical course and hospitalisation of these patients is illustrated in Box 3. Of the three patients remaining in ICU, two died (patient 13 and patient 6 in Box 3 on 8 August and 12 August, respectively), with the remaining patient discharged alive from hospital on 23 September 2009.
Box 2 shows that the mean duration of symptoms before presentation was 4 days, with fever, cough and dyspnoea being the most common symptoms in both groups. Similarly, there was no difference in the clinical signs between the two groups, with a diagnosis of pneumonia occurring in similar proportions of patients with seasonal and pandemic (H1N1) 2009 influenza.
Chest x-rays were taken of 59 of the 64 patients, and abnormalities were detected in 31 patients. Abnormal radiological features ranged from localised infiltrates to bilateral airspace consolidation (Box 4). Four patients had radiological abnormalities attributable to chronic lung conditions without evidence of concurrent pneumonia.
Fifty-three hospitalised patients were treated with a neuraminidase inhibitor and antibiotics. Corticosteroids were significantly more likely to be prescribed in the seasonal influenza group compared with the pandemic (H1N1) 2009 group (P < 0.05; Box 2). However, all corticosteroid use was deemed to be for treatment of an underlying respiratory illness rather than influenza. Treatment response did not differ significantly between the two groups, and the overall mean time to defervescence was 2.6 days (range, 0–10 days) and mean number of days in hospital was 8 days (range, 0–61 days). Overall, there were five deaths (four in the pandemic (H1N1) 2009 group and one in the seasonal influenza group). Two deaths occurred in the ICU (see above).
Significant morbidity and mortality from influenza in pregnant women during previous pandemics has been reported.10,11 For seasonal influenza, the highest morbidity for pregnant women occurred in the third trimester.12 There have been several reports that pregnant women are at increased risk of severe complications from pandemic (H1N1) 2009 influenza.5,6 However, we were unable to demonstrate a difference in clinical severity between the seasonal and the pandemic viruses. The reliability of our estimate is limited by the small sample size, and a definitive answer to this question requires a multicentre study.
The clinical and epidemiological characteristics of 18 people hospitalised with pneumonia caused by pandemic (H1N1) 2009 influenza in Mexico City have been reported.13 The age, presence of comorbid conditions, presenting symptoms, laboratory findings and clinical features were similar to those in our group. However, in the Mexican series the higher proportion of abnormalities on chest x-ray (all patients), the number of patients with severe disease requiring invasive ventilation (12), and the mortality rate (39%) differ from our series (mortality rate, 8%). This could reflect differing admission criteria and smaller numbers.
1 Admissions to Liverpool Hospital of 64 adults with confirmed seasonal and pandemic (H1N1) 2009 influenza during the “PROTECT” phase of the epidemic*
 |
2 Demographic, clinical and laboratory features of the 64 patients admitted to Liverpool hospital with seasonal and pandemic (H1N1) 2009 influenza, 17 June to 31 July 2009
3 Clinical courses of 13 patients admitted to Liverpool Intensive Care Unit (ICU) with pandemic (H1N1) 2009 influenza and seasonal influenza, 17 June to 31 July 2009*
 |
4 Chest x-rays showing typical abnormalities found in patients hospitalised with influenza, 17 June to 31 July 2009
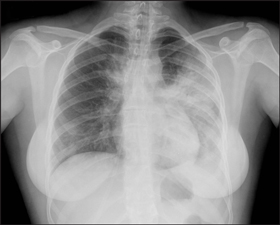 |
Patient with pandemic (H1N1) 2009 influenza showing airspace opacity in the left upper and left lower lobes and medial right upper zone. |
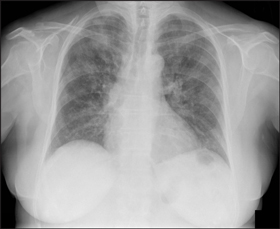 |
Patient with pandemic (H1N1) 2009 influenza showing bilateral interstitial and airspace opacity. |
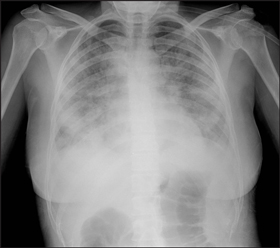 |
Patient with seasonal influenza showing bilateral airspace opacity. |
- Ya-Shu Chang1
- Sebastiaan J van Hal2
- Peter M Spencer1
- Iain B Gosbell3,2
- Peter W Collett1
- 1 Department of Respiratory Medicine, Liverpool Hospital, Sydney, NSW.
- 2 Department of Microbiology and Infectious Diseases, Sydney South West Pathology Service, Sydney, NSW.
- 3 Microbiology and Infectious Diseases Unit, School of Medicine, University of Western Sydney, Sydney, NSW.
We thank the medical and nursing staff of Liverpool Hospital, who assisted with the clinical care of patients affected by influenza, and the scientific staff of Sydney South West Pathology Service — Liverpool, who performed the diagnostic testing on the patients. We also thank Professor Guy Marks for his comments on our manuscript.
None identified.
- 1. Ginsberg M, Hopkins J, Maroufi A, et al. Swine influenza A (H1N1) infection in two children — southern California, March–April 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 400-402.
- 2. World Health Organization. Pandemic (H1N1) 2009. http://www.who.int/csr/disease/swineflu/en/index.html (accessed 6 Aug 2009).
- 3. Australian Government Department of Health and Ageing. Australian health management plan for pandemic influenza. 2008. http://www.flupandemic.gov.au/internet/panflu/publishing.nsf/Content/ahmppi-1 (accessed 6 Aug 2009).
- 4. Kaufman MA, Duke GJ, McGain F, et al. Life-threatening respiratory failure from H1N1 influenza 09 (human swine influenza). Med J Aust 2009; 191: 154-156. <MJA full text>
- 5. Fonseca V, Davis M, Wing R, et al. Novel influenza A (H1N1) virus infections in three pregnant women — United States, April–May 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 497-500.
- 6. Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374: 451-458.
- 7. Australian Government Department of Health and Ageing. H1N1 Influenza 09 latest news. New pandemic phase PROTECT. 17 June 2009. http://www.health.gov.au/internet/healthemergency/publishing.nsf/Content/news-170609 (accessed Nov 2009).
- 8. New South Wales Department of Health. Emergency. Pandemic influenza A (H1N1) 2009. Questions and answers. Common questions and answers regarding H1N1 Influenza 09. http://www.emergency.health.nsw.gov.au/swineflu/consumers/qa.asp (accessed 6 Aug 2009).
- 9. Centers for Disease Control and Prevention. Influenza. In: Heymann DL, editor. Control of communicable diseases manual. Washington, DC: American Public Health Association, 2008: 315-331.
- 10. Harris JW. Influenza occurring in pregnant women. JAMA 1919; 72: 978-980.
- 11. Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol 1959; 78: 1172-1175.
- 12. Neuzil KM, Reed GW, Mitchel EF, et al. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 1998; 148: 1094-1102.
- 13. Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med 2009; 361: 680-689. Epub 2009 Jun 29.





Abstract
Objective: To compare the patient characteristics, clinical features and outcomes of adult patients hospitalised with pandemic (H1N1) 2009 influenza and seasonal influenza.
Design and setting: Retrospective medical record review of all patients admitted to Liverpool Hospital, Sydney, with laboratory-confirmed influenza from the initiation of the “PROTECT” phase of the pandemic response on 17 June until the end of our study period on 31 July 2009.
Main outcome measures: Severity of illness; requirement for admission to the intensive care unit (ICU) and/or invasive ventilation; mortality.
Results: Sixty-four adults were admitted to Liverpool Hospital with influenza, 48 with pandemic (H1N1) 2009 influenza and 16 with seasonal influenza. Thirteen patients were admitted to the ICU. Seven required invasive ventilation, with 2 patients requiring ongoing extracorporeal membrane oxygenation (ECMO). Five patients died (mortality rate, 8%) with two deaths occurring after the study period. Patients with pandemic (H1N1) 2009 influenza were younger and less likely to be immunocompromised than patients with seasonal influenza. However, the clinical features of pandemic (H1N1) 2009 influenza and seasonal influenza were similar.
Conclusions: Our findings show that the clinical course and outcomes of pandemic (H1N1) 2009 influenza virus are comparable to those of the current circulating seasonal influenza in Sydney. The high number of hospital admissions reflects a high incidence of disease in the community rather than an enhanced virulence of the novel pandemic influenza virus.