Thrombolysis is a highly effective treatment for acute ischaemic stroke, when administered within 4.5 hours according to strict protocols.1,2 There have been concerns, however, that the results of clinical trials for stroke thrombolysis would be difficult to replicate in routine practice. The Safe Implementation of Thrombolysis in Stroke International Stroke Thrombolysis Register (SITS-ISTR) was established as a voluntary registry in over 700 centres around the world to address this concern.3 The SITS-ISTR had recruited over 30 000 patients by February 2010, including 704 Australian patients, and demonstrated clinical outcomes superior to those seen in clinical trials, including lower complication rates.4,5
The SITS registry may not reflect outcomes in the community. Centres registering cases on the SITS registry may have better outcomes. Although centres participating in the European Safe Implementation of Thrombolysis in Stroke Monitoring Study (SITS-MOST) were externally audited, there is no guarantee that centres subsequently participating in the SITS-ISTR report all cases.6 Stroke thrombolysis rates differ greatly between institutions and, to date, studies of thrombolysis for acute stroke have not been population based. The proportion of patients with acute stroke who receive thrombolysis has not been accurately estimated.7
We adapted previously developed principles for population-based stroke research8 to identify all cases of acute ischaemic stroke for which thrombolysis was administered from October 2007 to September 2009 in the entire state of South Australia, covering a population of 1.5 million people. We report the rates and types of intentional and accidental protocol violations in thrombolysis administration for stroke, and quantify the geographic disparity in thrombolytic therapy for stroke in urban and rural SA. We also examined differences between SITS-registered and unregistered cases.
A 2-year study period from 1 October 2007 to 30 September 2009 was chosen to coincide with the opening of an acute stroke unit (a total of three acute stroke units were operating during the study period). Multiple methods of case ascertainment were applied across urban, rural, public and private hospital systems to identify all cases of acute stroke for which thrombolysis was administered. SA is geographically isolated from other states, so no patients with acute stroke in SA are transferred interstate. Ethics approval was granted by the ethics committees of the Queen Elizabeth Hospital, Royal Adelaide Hospital and Flinders Medical Centre.
Case notes were examined by one of us (J M L) for time of symptom onset, time of arrival at hospital, whether the patient had been transferred from another hospital and time of thrombolysis administration.
Symptomatic intracranial haemorrhage was defined as any recorded haemorrhage with altered neurological findings (the National Institute of Neurological Disorders and Stroke [NINDS] definition9). The NINDS definition was the most suitable for a retrospective audit of case notes and is likely to overestimate symptomatic haemorrhage compared with other definitions.4 All imaging was reviewed by one of us (W K C) and haemorrhage was classified using the European Cooperative Acute Stroke Study (ECASS) grading system.10 Finally, the death register was examined for ascertained cases.
The accepted protocol for the administration of thrombolysis was that described in the 2007 National Stroke Foundation guidelines.11 Following the publication of results from the ECASS-III trial in September 2008, the allowable time window for administration was extended from 3 hours to 4.5 hours.1 Evidence of exclusion criteria in case notes was recorded. If the notes mentioned that the treating medical team was aware of a particular protocol violation but decided to administer the medication anyway, the violation was regarded as intentional. The reported final diagnosis for the administration of thrombolysis was also recorded.
Over the 2-year study period, 158 instances of thrombolytic therapy for suspected acute ischaemic stroke in SA were identified in 157 patients; 84 of these patients were male and 73 were female. The median age of patients was 76 years (interquartile range, 71–79 years), and 43 patients (27%) were aged over 80 years. One patient was an Indigenous Australian.
Eighteen of the 157 patients who received thrombolysis (11%) initially presented to hospitals that do not have stroke units — five received thrombolysis after transfer to a hospital with a stroke unit, and 13 (8%) received thrombolysis in hospitals without stroke units. More than half of the admissions for stroke in SA recorded by Medicare from June 2006 to June 2008 were to hospitals without acute stroke units.
The 158 cases of stroke for which thrombolysis was administered were ascertained as summarised in Box 1. A total of 8914 admissions to urban public hospitals in SA that related to any patient coded with any form of stroke or transient ischaemic attack were identified, for which 8201 discharge summaries were available (92%). From the discharge summaries it was determined that stroke was the principle reason for, or occurred during, 4106 admissions, and 916 of these admissions were rehabilitation admissions or interhospital transfers. Of the remaining 3190 admissions, 2485 hospital admissions represented acute presentations with ischaemic stroke. Ischaemic stroke occurred while the patient was in hospital in 76 of the admissions. Thrombolytic therapy for stroke was identified in 145 hospital admissions in 144 patients from discharge summaries (one patient received thrombolysis twice during the study period).
Of 72 082 brain scans (computed tomography and magnetic resonance imaging) performed in Adelaide public hospitals during the study period, 136 instances of thrombolysis in 135 patients were identified. Of 131 064 public hospital encounters, 50 cases were identified where thrombolysis for stroke had been reported as a procedure during the study period. From pharmacy dispensing records for thrombolytic drugs, 28 cases were identified (thrombolytic drugs were frequently dispensed without patient identifiers).
Seventy-two patients had been recruited into the SITS registry, all of whom were also ascertained by other means. Within the two hospitals participating in the SITS registry, 15 eligible cases were identified in patients who had not been enrolled into the SITS registry. The prospective population-based stroke study detected eight patients, all of whom were also ascertained by other means.
Private hospital thrombolysis. Four patients received thrombolysis in the private sector; each was identified by both the treating neurologist and the hospital coordinator. Medicare recorded 782 private hospital admissions for stroke from June 2006 to June 2008.
Patients residing outside Adelaide. Eleven patients residing outside the urban region of Adelaide received thrombolysis over the study period, of whom two received thrombolysis while visiting Adelaide and five were taken directly to urban hospitals. The population outside Adelaide comprises 26% of the total South Australian population (394 000 of 1.5 million) but comprised only 7% of all stroke thrombolysis cases (P < 0.001). Over the 2-year period from June 2006 to June 2008, Medicare recorded 997 stroke admissions in hospitals outside Adelaide, out of a total of 3521 for SA (28%), suggesting that stroke incidence is at least as high in these areas as it is in urban Adelaide.
Symptom-to-treatment times. Symptom onset time could not be determined for 10 cases. For the remaining patients, the median time from symptom onset to hospital arrival was 55 minutes. In 21 cases, thrombolysis was administered after a stroke that occurred in hospital. In 11 cases, the hospital arrival time or time of thrombolytic therapy could not be identified from the notes. For the remaining patients, the median time from arrival to thrombolysis was 85 minutes, which was significantly greater (P < 0.001) than the 60 minutes recommended by the American Heart Association.12
Protocol violation and misdiagnoses. In five cases, thrombolytic therapy was administered when absolute contraindications existed (Box 2). In one of these cases the patient had a fatal intracerebral haemorrhage after an asymptomatic subacute infarction was missed in the initial radiological review, and in three the treating physician was aware of the contraindication. In 53 cases, thrombolytic therapy was administered when at least one relative contraindication existed (Box 2). In all these cases the patient was aged over 80 years.
In four cases, the diagnosis was not stroke. One of these diagnoses was Bell’s palsy with psychogenic features, one was hemiplegic migraine, and two cases were deemed psychogenic presentations.
Haemorrhage after thrombolysis. All haemorrhages occurred within 36 hours of thrombolysis. Fifteen patients (10%) had symptomatic intracranial haemorrhage. This did not differ significantly from the 6.4% rate recorded in the NINDS trial (P = 0.30).9 Haemorrhage rates apparent on imaging, defined using the ECASS classification, are shown in Box 3.
Distance from acute stroke unit. For each postcode, the number of patients who received thrombolysis per 100 000 population was plotted against the distance from the nearest acute stroke unit (Box 4), and a strong inverse relationship was observed between these variables. There was no relationship between average or median age and distance from the nearest acute stroke unit.
Difference between SITS-registered and unregistered cases. Seventy-two of the 158 cases were entered in the SITS registry (Box 5). There was no significant difference in demographics or bleeding complications between those entered and not entered into the SITS registry. Cases not entered had longer arrival-to-treatment times (P < 0.01) and longer symptom-to-treatment times, including cases in which the stroke occurred in hospital (P < 0.001). This analysis excluded patients treated outside urban Adelaide.
Proportion of stroke admissions that involved thrombolysis. During the study period, 4% (155/4106) of acute hospital admissions for stroke in urban public hospitals involved administration of thrombolysis. Eight patients received thrombolysis outside the urban public hospital system. Medicare estimates from 2006 to 2008 suggest that over 50% of acute stroke admissions in SA occur outside the urban public hospital system, hence the proportion of acute strokes in SA for which thrombolysis is administered is less than 2%. However, for patients residing within 5 km of an acute stroke unit, the thrombolysis rate for stroke was just over 10% (65 patients in 647 strokes).
This study was possible due to unique clinical data collection methods and cooperative hospital relationships. Case ascertainment was comprehensive and validated against coexisting registers and a population stroke study. Although the numbers are small, there was no difference in haemorrhage rates between the 72 cases registered in the SITS trial and the 86 which were not. Patients registered in the SITS trial received treatment about 30 minutes sooner, excluding rural cases. With larger numbers, such a difference in time to treatment could result in different outcomes, suggesting that outcomes for stroke thrombolysis in the SITS registry may be better than those in the broader community.13
The rate of symptomatic intracranial haemorrhage was not significantly different from the rate reported for the NINDS study (10% v 6.4%). A third of patients received thrombolysis despite contraindications defined by the National Stroke Foundation guidelines.11 In a Finnish study of more than 1000 stroke patients who were treated with thrombolysis, half were treated outside guidelines — suggesting that most contraindications may be unnecessary, or at least “relative” contraindications.14 Haemorrhage rates apparent on imaging were similar to those reported from previous studies.10
In a 2007 prospective hospital-based study of 259 stroke patients admitted to a tertiary hospital in Adelaide, it was estimated that 16% of stroke patients were potentially eligible for thrombolysis and arrived within the treatment time for thrombolysis.15 A much larger proportion would have been eligible had they arrived sooner. Currently, many stroke patients who would benefit from thrombolysis do not receive it. Similar data have been reported throughout the Western world. In the United States, it is estimated from Medicare data alone that fewer than 3% of stroke patients admitted to hospital receive thrombolysis.16 In the United Kingdom, a national audit showed that 1.4% of stroke patients were treated with thrombolysis, but some individual centres achieved much higher thrombolysis rates.7
Distance is an obstacle to thrombolysis for stroke in SA, even within metropolitan Adelaide. In our study, residing more than five kilometres from an acute stroke unit diminished the chance of receiving thrombolytic therapy — this distance is only a matter of minutes by car in Adelaide. At a distance of more than about five kilometres from an acute stroke unit, a patient is much more likely to be taken to a closer alternative hospital. Despite over half of stroke admissions occurring at hospitals without stroke units, transferred patients represented only 3% of those who received thrombolysis. Also, although age is a strong risk factor for stroke,17 there was no relationship between age and distance from acute stroke unit.
The barriers to thrombolysis for stroke in rural areas, including lack of rapid imaging and medical expertise, are formidable; yet a small number of stroke patients do receive thrombolysis in the rural health system. Rural doctors come under pressure to provide treatment options to their patients. As unsupported administration of thrombolysis for stroke is not recommended in Australian guidelines,18 Australia must develop networks that link rural doctors with stroke physicians.
The shortfalls in stroke service delivery in SA are currently being addressed by the Stroke Clinical Network, established by SA Health in 2009. Offering thrombolysis to all eligible patients is a main objective. Strategies include paramedic stroke identification, patient redirection to stroke units, acute stroke management protocols, and rural stroke service networks. Unified hospital coding of thrombolysis is being implemented to monitor the success of these strategies.
Our results show that a large proportion of patients in SA are missing out on acute stroke therapy as a result of poor access to acute stroke units in urban and rural settings. Australia must face the challenge of delivering stroke thrombolysis safely and frequently.
1 Ascertainment of cases of acute stroke in South Australia for which thrombolysis was administered, October 2007 to September 2009*
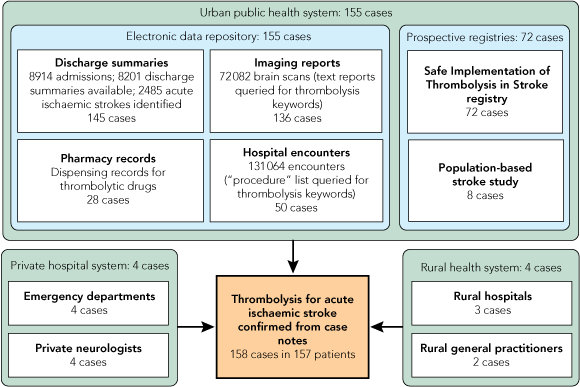 * Only three cases were ascertained by one method alone. |
2 Protocol violations according to the 2007 National Stroke Foundation guidelines11
|
No. of patients |
Intended by treating doctors |
Symptomatic haemorrhage |
||||||||||||
Absolute contraindications |
|
|
|
||||||||||||
Stroke symptoms on waking (time of onset unknown) |
1 |
Yes |
No |
||||||||||||
International normalised ratio of 1.9 before administration |
1 |
No |
No |
||||||||||||
Reported seizure of unaffected side |
1 |
Yes |
No |
||||||||||||
Retrospective radiological review showed subacute ischaemic stroke on contralateral side |
1 |
No |
Yes* |
||||||||||||
No imaging performed before administration in rural setting |
1 |
Yes |
No |
||||||||||||
Relative contraindications |
|
|
|
||||||||||||
National Institutes of Health Stroke Scale score > 22 |
1 |
Yes |
No |
||||||||||||
Large established cerebral infarction on preliminary CT scan |
1 |
No |
No |
||||||||||||
Recent major surgery |
2 |
Yes |
Yes† |
||||||||||||
Patient aged over 80 years |
53 |
Yes |
10‡ |
||||||||||||
CT = computed tomography. * Fatal intracerebral haemorrhage. † Both patients had bleeding at surgical site, and both survived. ‡ Seven patients had intracerebral haemorrhages, of whom five died. |
|||||||||||||||
3 Occurance of haemorrhage within 36 hours of thrombolysis based on imaging, according to the European Cooperative Acute Stroke Study grading system10
Classification |
No. |
||||||||||||||
Parenchymal haematoma type 2 (PH2): a dense haematoma in > 30% of the infarcted area with substantial space-occupying effect, or any haemorrhagic lesion outside the infarcted area) |
21 |
||||||||||||||
Parenchymal haematoma type 1 (PH1): a haematoma in ≤ 30% of infarcted area with some slight space-occupying effect |
9 |
||||||||||||||
Haemorrhagic infarction type 2 (HI2): confluent petechiae within the infarcted area but without space-occupying effect |
7 |
||||||||||||||
Haemorrhagic infarction type 1 (HI1): small petechiae along the margins of the infarct |
2 |
||||||||||||||
No haemorrhage visible |
116 |
||||||||||||||
Imaging not performed or imaging not available |
3 |
||||||||||||||
4 Number of patients who received thrombolysis for acute stroke per 100 000 population according to distance from an acute stroke unit*
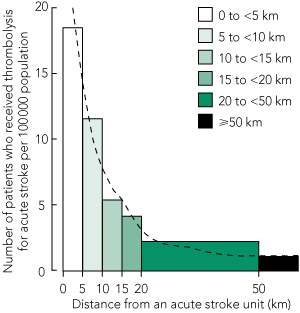 * The dashed line represents locally weighted regression applied to individual postcode data. |
5 Comparison of SITS-registered and unregistered cases
|
Registered (n = 72) |
Unregistered (n = 86) |
|||||||||||||
Average age (years) |
72 |
72 |
|||||||||||||
Symptomatic intracranial haemorrhage |
8 |
7 |
|||||||||||||
Symptomatic extracranial haemorrhage |
3 |
4 |
|||||||||||||
Death from intracranial haemorrhage |
4 |
4 |
|||||||||||||
Diagnosis other than stroke |
0 |
4 |
|||||||||||||
Thrombolysis protocol violation — absolute contraindication* |
1 |
4 |
|||||||||||||
Thrombolysis protocol violation — relative contraindication* |
27 |
26 |
|||||||||||||
Median symptom-to-arrival time (min) |
53 |
56 |
|||||||||||||
Median arrival-to-treatment time (min) |
74 |
95† |
|||||||||||||
Median symptom-to-treatment time (min) |
131 |
165‡ |
|||||||||||||
|
SITS = Safe Implementation of Thrombolysis in Stroke. * One or more contraindications according to the 2007 National Stroke Foundation guidelines.11 † P < 0.01. ‡ P < 0.001. |
|||||||||||||||
Received 14 July 2010, accepted 12 October 2010
- James M Leyden1
- Woon K Chong1
- Tim Kleinig2
- Andrew Lee3
- John B Field4
- Jim Jannes1
- 1 Queen Elizabeth Hospital, Adelaide, SA.
- 2 Royal Adelaide Hospital, Adelaide, SA.
- 3 Comprehensive Stroke Centre, Flinders Medical Centre, Adelaide, SA.
- 4 Faculty of Health Sciences, University of Adelaide, Adelaide, SA.
We thank Lizzie Dodd, Stroke Nurse, Queen Elizabeth Hospital, for help in reviewing SITS cases; Grant Emmerson, Manager, Analysis and Reporting, Information Communication and Technology Delivery Services, SA Health, for help in developing searches within public hospital data repositories; and Clarabelle Pham and Jonathan Karnon, Risk Adjusted Cost-Effectiveness (RAC-E) team, University of Adelaide, for providing Medicare data.
None identified.
- 1. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363: 768-774.
- 2. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317-1329.
- 3. Kaste M, Thomassen L, Grond M, et al. Thrombolysis for acute ischemic stroke: a consensus statement of the 3rd Karolinska Stroke Update, October 30–31, 2000. Stroke 2001; 32: 2717-2718.
- 4. Wardlaw JM, Murray V, Berge E, Del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2009; (4): CD000213.
- 5. Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase 3-4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet 2008; 372: 1303-1309.
- 6. Leung TW, Wong KS. Thrombolysis with alteplase for acute ischemic stroke: safe and effective outside the 3-hour time window? Nat Clin Pract Neurol 2009; 5: 70-71.
- 7. Sudlow C, Warlow C. Getting the priorities right for stroke care. BMJ 2009; 338: b2083.
- 8. Sudlow CL, Warlow CP. Comparing stroke incidence worldwide: what makes studies comparable? Stroke 1996; 27: 550-558.
- 9. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581-1587.
- 10. Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999; 30: 2280-2284.
- 11. National Stroke Foundation. Clinical guidelines for acute stroke management. Melbourne: NSF, 2007.
- 12. Fonarow GC, Reeves MJ, Smith EE, et al. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in Get With The Guidelines-Stroke. Circ Cardiovasc Qual Outcomes 2010; 3: 291-302.
- 13. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375: 1695-1703.
- 14. Meretoja A, Putaala J, Tatlisumak T, et al. Off-label thrombolysis is not associated with poor outcome in patients with stroke. Stroke 2010; 41: 1450-1458.
- 15. Kleinig TJ, Kimber TE, Thompson PD. Stroke prevention and stroke thrombolysis: quantifying the potential benefits of best practice therapies. Med J Aust 2009; 190: 678-682. <MJA full text>
- 16. Kleindorfer D, Xu Y, Moomaw CJ, et al. US geographic distribution of rt-PA utilization by hospital for acute ischemic stroke. Stroke 2009; 40: 3580-3584.
- 17. Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2003; 2: 43-53.
- 18. Ad Hoc Committee representing the National Stroke Foundation and the Stroke Society of Australasia. The implementation of intravenous tissue plasminogen activator in acute ischaemic stroke — a scientific position statement from the National Stroke Foundation and the Stroke Society of Australasia. Intern Med J 2009; 39: 317-324.





Abstract
Objective: To report the rate of thrombolysis for treating acute stroke in South Australia from October 2007 to September 2009. We hypothesised that the rate of thrombolytic therapy would be related to distance from an acute stroke unit.
Design, setting and patients: An observational, population-based, retrospective review of case notes and imaging, using multiple case-ascertainment methods. Patients administered a thrombolytic agent by any method for suspected ischaemic stroke in urban, rural, public and private hospitals in SA (covering a population of 1.5 million people) were included.
Main outcome measures: Absolute and relative contraindications for thrombolysis administration in each case, according to the 2007 National Stroke Foundation guidelines; incidence of haemorrhage; and population thrombolysis rates according to distance from an acute stroke unit.
Results: A total of 158 cases of thrombolytic therapy for suspected acute ischaemic stroke were identified in 157 patients. Fifteen patients (10%) had symptomatic intracranial haemorrhage, of whom eight (5%) died within 3 months. Seven patients had symptomatic extracranial haemorrhage. Five patients (3%) received thrombolysis despite absolute contraindications. Patients living closer to stroke units were more likely to receive thrombolysis.
Conclusions: Rates of symptomatic haemorrhage after thrombolysis were similar to those in voluntary registries. A large proportion of South Australians are currently missing out on acute stroke therapy as a result of poor access to acute stroke units in both urban and rural settings. It is estimated that fewer than 2% of ischaemic stroke patients are administered thrombolysis in SA.