Stroke is common, and frequently fatal or disabling.1,2 In Australia, stroke is the second leading contributor to disease burden after ischaemic heart disease.3
Stroke occurs most commonly in patients with known risk factors. It has been estimated that the proportion attributable to these factors is as high as 80%,4 implying that stroke is largely a preventable disease. However, as various preventive therapies are now widely employed, it is uncertain whether these estimates still apply.
The effects of ischaemic stroke (the most common kind) can potentially be minimised with thrombolysis.5 However, thrombolysis rates in Australia are around 3%,6 and treatment rates, even at centres of excellence, struggle to exceed 10%.7 There is therefore scope for reducing the effects of stroke by expanding thrombolysis.
The Royal Adelaide Hospital serves a local population within metropolitan Adelaide, and is also a tertiary referral centre for adjacent rural areas. Its stroke unit admits all patients with a diagnosis of acute stroke regardless of age or severity, except those requiring neurosurgical intervention (subarachnoid haemorrhage and some with intracerebral haemorrhage) and those transferred from high-level residential care. The study was approved by the Royal Adelaide Hospital Research Ethics Committee.
We calculated percentage stroke preventability using a previously published method,8 assuming a multiplicative scale for relative risk reductions of underutilised therapies:
Factors assessed included untreated or suboptimally treated hypertension,9 untreated or suboptimally treated atrial fibrillation,10 indications for statin11 or antiplatelet therapy but no treatment,12 inappropriate hormone replacement therapy,13 untreated “normal” post-stroke blood pressure,14 untreated symptomatic carotid disease,15 smoking,16 and subtherapeutic warfarinisation,17 (Box 1). Only risk factors with strong data from randomised controlled trial (RCT) meta-analyses or large RCTs were included, except for smoking,16 which has robust population relative risk data, and for suboptimal anticoagulation, which cannot feasibly be assessed in an RCT but clearly increases stroke risk.17,18 Studies on the effect of smoking and hypertension on stroke risk have not always reliably distinguished ischaemic and haemorrhagic stroke, and hence the impact of these factors on the preventability of ischaemic and haemorrhagic stroke was not differentially weighted.
Efficacy of hypertension management was assessed by comparing the last two prestroke systolic blood pressure readings with national guidelines.19 Undertreated hypertension was identified where both readings were more than 10 mmHg above those recommended in guidelines, and grossly undertreated hypertension was identified where both were more than 20 mmHg above. Only systolic pressure was considered.20 Adequacy of anticoagulation for atrial fibrillation was assessed by comparison with national guidelines,21 and hypercholesterolaemia with Pharmaceutical Benefits Scheme guidelines applicable in 2006. Hormone replacement therapy was designated inappropriate unless a patient had significant menopausal symptoms refractory to other therapies. Inadequate warfarininsation was defined as subtherapeutic admission and preadmission international normalised ratio (INR) readings or, in the case of periprocedural stroke, a failure to follow national guidelines.22 All smoking-associated strokes were deemed potentially preventable.
Potential prevention of disability from stroke by thrombolysis was assessed by comparing actual versus potential thrombolysis cases (ie, patients with ischaemic stroke potentially able to reach hospital within 120 minutes of stroke onset with no contraindications). A number needed to treat (NNT) of 8 (95% CI, 5.3–15.3) was derived from a recent meta-analysis,5 being the number needed to render one patient who had had a stroke minimally symptomatic or asymptomatic (a modified Rankin score of ≤ 1).
Demographic data, risk factor prevalence and therapies, and stroke characteristics are shown in Box 2. Almost all patients identified a treating doctor. Stroke type and mortality, median ages and sex distribution did not differ from recent Australian population-based studies.2,23 Over 70% of patients were being treated with at least one preventive therapy. The most common previously identified risk factor was hypertension. Most patients with hypertension were being treated, many with polytherapy.
Overall, 135 patients (52%) had at least one suboptimally modified risk factor (Box 3), and 10% had two or more. In these patients, average stroke preventability was 52%. Therefore, overall, 70 out of 259 strokes (27%) were preventable.
At least two recent blood pressure measurements had been recorded for 95% of patients; a quarter were not within the target range. Patients were more likely to have had inadequately controlled hypertension if their target systolic pressure was 130 mmHg or less (patients with diabetes, young-onset hypertension and renal disease;19 relative risk, 4.27; 95% CI, 2.58–7.05; P < 0.001).
We had hypothesised that the very old were more likely to be treated suboptimally. However, as shown in Box 4, we found the opposite. There was a strong trend for the association of suboptimally managed risk factors with younger age, principally because of the prevalence of smoking and unmet blood pressure targets in this group. Our other hypotheses, that rural address, social isolation, non-English-speaking background and being male might be linked to stroke preventability, were not supported.
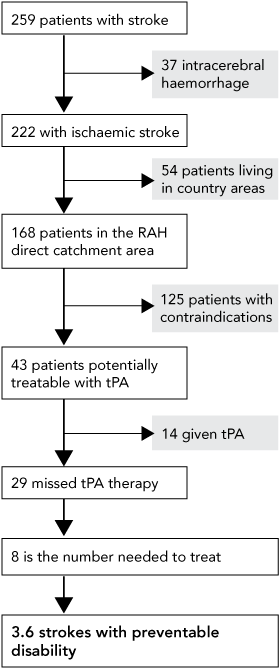
Compared with optimal modification of risk factors, fewer strokes were “preventable” with best-practice thrombolysis (Box 5). Roughly a quarter of stroke patients were ineligible for treatment with tissue plasminogen activator because of intracerebral haemorrhage, or were not transferred in time from rural hospitals. Of the remaining 168, 125 had a contraindication to thrombolysis, most commonly because of time-of-onset uncertainty (most often awaking with neurological symptoms) or unavoidable presentation outside the 3-hour window (eg, being found by relatives after a prolonged period). A third of potential thrombolysis (14/42) candidates received treatment. Assuming a number needed to treat of 8,5 disability could potentially have been prevented in four strokes under optimal conditions.
Estimates of stroke preventability were conservative, as lifestyle factors contributing independently to stroke risk that cannot be readily quantified were not included (eg, obesity,24 physical inactivity,25 alcohol consumption26 and poor diet24). Furthermore, statin therapy,27 therapeutic anticoagulation18 and angiotensin-converting enzyme inhibition28 not only prevent stroke, but also lessen stroke severity.
Stroke preventability is difficult to define and quantify, and our finding that a quarter of strokes could be prevented is less than the 80% attributable risk cited in the introduction,4 and a recently estimated 80% reduction in recurrence risk with best-practice treatments.8 However, our population was already being treated with a number of preventive therapies, in contrast to patients in both cited articles, in which no therapy, rather than some therapy was assumed. Additionally, one article focused only on secondary prevention, and included dietary and exercise interventions,8 which are not proven on a population basis for primary stroke prevention. Our study identifies and quantifies the risk factors that remain suboptimally managed in an era of widespread medical therapies and declining smoking habits.
We found that the three suboptimally modified risk factors with the greatest impact were smoking, hypertension and atrial fibrillation. This finding is consistent with a recent population-based study of general practice patients,29 and broadly consistent with international data.30 Smoking and suboptimally treated hypertension were more prevalent in younger patients, explaining the higher prevalence of “stroke preventability” in this group. These patients often also had lower blood pressure target values, which could only have been achieved by combination therapy. Hypertension and other risk factors were well managed in older patients, despite the greater prevalence of risk factors, refuting the possibility that therapeutic nihilism or undertreatment among older people may be a significant cause of ischaemic stroke. We did, however, demonstrate a continued reluctance of health practitioners to prescribe warfarin and of patients to take it, also documented elsewhere.31 It is to be hoped that new data confirming the efficacy and safety of warfarin therapy in older people will help allay persisting concerns.32
1 Relative risk reductions from published studies for modification of designated risk factors for stroke
* 95% confidence intervals were not provided in the articles cited. |
|||||||||||||||
2 Demographic characteristics, prestroke risk factors and therapies, and stroke characteristics and outcomes for 259 patients admitted to Royal Adelaide Hospital stroke unit from 24 January 2006 to 10 January 2007
Median admission National Institutes of Health stroke scale score |
|||||||||||||||
3 Total strokes preventable by optimal management of each risk factor for 135 patients with one or more risk factors
- Timothy J Kleinig1
- Thomas E Kimber1
- Philip D Thompson1,2
- 1 Royal Adelaide Hospital, Adelaide, SA.
- 2 University of Adelaide, Adelaide, SA.
Timothy Kleinig received funding from Servier (manufacturer of perindopril) to attend the Australasian stroke conference in Perth in 2007.
- 1. Sturm JW, Dewey HM, Donnan GA, et al. Handicap after stroke: how does it relate to disability, perception of recovery, and stroke subtype? The North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2002; 33: 762-768.
- 2. Thrift AG, Dewey HM, Macdonell RA, et al. Stroke incidence on the east coast of Australia: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2000; 31: 2087-2092.
- 3. Mathers CD, Vos ET, Stevenson CE, Begg SJ. The Australian Burden of Disease Study: measuring the loss of health from diseases, injuries and risk factors. Med J Aust 2000; 172: 592-596. <MJA full text>
- 4. Hankey GJ. Potential new risk factors for ischemic stroke: what is their potential? Stroke 2006; 37: 2181-2188.
- 5. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363: 768-774.
- 6. Cadilhac D, Hankey G, Harris D, et al on behalf of the National Stroke Foundation and the National Stroke Audit Collaborative. National stroke audit clinical report acute services. Melbourne: NSF, 2007. http://www.strokefoundation.com.au/images/stories/healthprofession als/national%20stroke%20audit%20clinical %20report%20acute%20services.pdf (accessed May 2009).
- 7. Bray JE, Coughlan K, Bladin C. Thrombolytic therapy for acute ischaemic stroke: successful implementation in an Australian tertiary hospital. Intern Med J 2006; 36: 483-488.
- 8. Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke 2007; 38: 1881-1885.
- 9. Chalmers J, Todd A, Chapman N, et al. International Society of Hypertension (ISH): statement on blood pressure lowering and stroke prevention. J Hypertens 2003; 21: 651-663.
- 10. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146: 857-867.
- 11. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 2005; 366: 1267-1278.
- 12. Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324: 71-86.
- 13. Magliano DJ, Rogers SL, Abramson MJ, Tonkin AM. Hormone therapy and cardiovascular disease: a systematic review and meta-analysis. BJOG 2006; 113: 5-14.
- 14. PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358: 1033-1041.
- 15. Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003; 361: 107-116.
- 16. Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk 1999; 6: 207-211.
- 17. Reynolds MW, Fahrbach K, Hauch O, et al. Warfarin anticoagulation and outcomes in patients with atrial fibrillation: a systematic review and meta-analysis. Chest 2004; 126: 1938-1945.
- 18. Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med 2003; 349: 1019-1026.
- 19. National Heart Foundation of Australia. Hypertension management guide for doctors. Canberra: NHFA, 2003.
- 20. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke 2004; 35: 776-785.
- 21. Hankey GJ, on behalf of the National Blood Pressure Advisory Committee of the National Heart Foundation. Non-valvular atrial fibrillation and stroke prevention [position statement]. Med J Aust 2001; 174: 234-239. <MJA full text>
- 22. Baker RI, Coughlin PB, Gallus AS, et al; the Warfarin Reversal Consensus Group. Warfarin reversal: consensus guidelines, on behalf of the Australasian Society of Thrombosis and Haemostasis. Med J Aust 2004; 181: 492-497. <MJA full text>
- 23. Hardie K, Jamrozik K, Hankey GJ, et al. Trends in five-year survival and risk of recurrent stroke after first-ever stroke in the Perth Community Stroke Study. Cerebrovasc Dis 2005; 19: 179-185.
- 24. Curioni C, André C, Veras R. Weight reduction for primary prevention of stroke in adults with overweight or obesity. Cochrane Database Syst Rev 2006; (4): CD006062.
- 25. Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke 2003; 34: 2475-2481.
- 26. Reynolds K, Lewis B, Nolen JD, et al. Alcohol consumption and risk of stroke: a meta-analysis. JAMA 2003; 289: 579-588.
- 27. Marti-Fabregas J, Gomis M, Arboix A, et al. Favorable outcome of ischemic stroke in patients pretreated with statins. Stroke 2004; 35: 1117-1121.
- 28. Chitravas N, Dewey HM, Nicol MB, et al. Is prestroke use of angiotensin-converting enzyme inhibitors associated with better outcome? Neurology 2007; 68: 1687-1693.
- 29. Sturm JW, Davis SM, O’Sullivan JG, et al. The Avoid Stroke as Soon as Possible (ASAP) general practice stroke audit. Med J Aust 2002; 176: 312-316. <MJA full text>
- 30. Rother J, Alberts MJ, Touze E, et al. Risk factor profile and management of cerebrovascular patients in the REACH Registry. Cerebrovasc Dis 2008; 25: 366-374.
- 31. Darkow T, Vanderplas AM, Lew KH, et al. Treatment patterns and real-world effectiveness of warfarin in nonvalvular atrial fibrillation within a managed care system. Curr Med Res Opin 2005; 21: 1583-1594.
- 32. Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370: 493-503.





Abstract
Objective: To identify and quantify current deficiencies in primary and secondary stroke prevention, as well as potential gains from optimal employment of thrombolysis.
Design, participants and setting: Observational study of 259 consecutive patients admitted to a tertiary hospital stroke unit from 24 January 2006 to 10 January 2007, with retrospective assessment of prestroke risk factors and therapies to determine stroke preventability, based on relative risk reductions from published meta-analyses of preventive therapies.
Main outcome measures: Numbers of strokes preventable by optimal risk factor modification and numbers of strokes with preventable disability through optimal thrombolysis; characteristics of patients with preventable strokes; contributions of each risk factor to stroke preventability.
Results: 183 patients had a disabling or fatal stroke; 135 patients had at least one suboptimally managed risk factor. On the basis of prespecified stroke preventability weightings, 70 strokes were preventable. The younger the patient, the more likely that the stroke was potentially preventable (relative risk [RR] for age < 60: ≥ 80 years, 3.10; 95% CI, 1.96–4.92). Smoking, inadequate control of hypertension and suboptimal anticoagulation accounted for nearly 90% of preventable strokes. Patients with target systolic blood pressures of 130 mmHg or lower were more likely to have inadequately controlled hypertension (RR, 4.27; 95% CI, 2.58–7.05). By comparison, disability could have been prevented in four strokes through optimal thrombolysis.
Conclusions: A significant proportion of stroke remains preventable, especially in younger patients, by optimal modification of risk factors, particularly smoking, blood pressure and anticoagulation. Only a small proportion of patients will benefit from best-practice thrombolysis.