Pneumococcal disease is estimated to cause over 800 000 child deaths a year worldwide.1 In areas with universal childhood vaccination programs using the 7-valent pneumococcal conjugate vaccine (7vPCV), the incidence of invasive pneumococcal disease (IPD) in young children has significantly decreased.2-4 In Australia, 7vPCV has been included in the childhood immunisation program for Indigenous children since mid 2001. This was subsequently expanded to include all Australian children in January 2005. The overall incidence of IPD in Australian children under the age of 2 years is reported to have declined by 75% between 2002 and 2006.5
7vPCV covers seven pneumococcal serotypes (4, 6B, 9V, 14, 18C, 19F and 23F). Before its use, these seven serotypes accounted for 84% of IPD cases in Australian children younger than 5 years.6 While 7vPCV use has led to a decrease in the overall incidence of IPD, studies in the United States,2,7 Canada8 and Spain9,10 have reported the emergence of IPD caused by serotypes not covered by 7vPCV, particularly serotype 19A. In contrast, several other studies found no increase in IPD caused by non-vaccine serotypes in areas where routine 7vPCV vaccination is carried out.11-13
The emergence of serotype 19A is clinically important, as it is prevalent worldwide and has shown multidrug resistance.14,15 From 2001 to 2007, the incidence of IPD caused by serotype 19A among non-Indigenous children aged less than 5 years in Western Australia increased from 1.6 to 7.8 per 100 000.16 This increase was not observed among Indigenous children, and a study of Indigenous people in Queensland also found no evidence for an increase in IPD caused by serotype 19A.17 Notably, these two studies were confined to individual states of Australia. As such, a nationwide study may provide additional power to detect changes and trends in IPD and serotype distribution.
We evaluated the incidence of IPD in Australian children under 2 years of age from 2002 to 2007 to determine the dynamics of both 7vPCV and non-7vPCV serotype IPD, the effects of the widespread introduction of 7vPCV, and differences in the dynamics of serotype 19A in Indigenous and non-Indigenous children on a national level.
IPD has been a notifiable disease in all Australian jurisdictions since 2001. Information is collected by the National Notifiable Diseases Surveillance System (NNDSS), with complete information from all states and territories available from 2002 onwards. We extracted data from the NNDSS in April 2009, including age, Indigenous status and serotype of the pneumococcal isolate. Our case definition was in accordance with the guidelines set by the NNDSS, where IPD is determined by the identification of Streptococcus pneumoniae through culture or nucleic acid testing from any normally sterile site.
We obtained data on the estimated resident population from the Australian Bureau of Statistics (ABS).18 Estimates of the Indigenous Australian population were based on ABS estimates for 2002 to 2006 and projected data for 2007.19 The population of non-Indigenous children was calculated by subtracting the estimate of the Indigenous population from the estimated resident population. Incidence rates were calculated using the respective population estimates for each year as the denominator.
Between 2002 and 2007, there were 1871 IPD case notifications recorded by the NNDSS among all Australian children aged < 2 years (148 in Indigenous children, 1441 in non-Indigenous children, and 282 in children whose Indigenous status was unknown). Serotype was determined in 1629 cases (87%). As shown in Box 1, the incidence of IPD decreased by 74% between 2002 and 2007 (from 98.1 to 25.1 per 100 000, P < 0.001). The reduction in IPD incidence was more pronounced in non-Indigenous children (from 84.7 to 19.5 per 100 000, P < 0.001) than Indigenous children (from 129.1 to 82.3 per 100 000, P < 0.05).
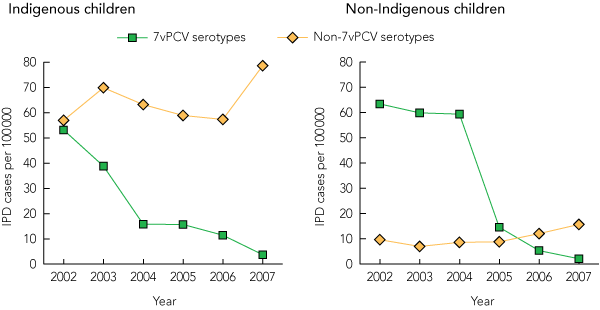
IPD cases caused by 7vPCV serotypes decreased overall by 97%, accounting for 9% of IPD cases in 2007 compared with 71% of cases in 2002. Box 2 compares the change in incidence of IPD caused by 7vPCV and non-7vPCV serotypes in Indigenous and non-Indigenous children aged < 2 years from 2002 to 2007. The incidence of IPD caused by 7vPCV serotypes decreased significantly among both Indigenous children (from 53.2 to 3.7 per 100 000, P < 0.001) and non-Indigenous children (from 63.4 to 2.1 per 100 000, P < 0.001). The incidence of IPD caused by non-7vPCV serotypes did not change significantly among Indigenous children (P = 0.60). In contrast, the incidence of non-7vPCV serotype IPD among non-Indigenous children increased significantly (from 9.7 to 15.7 per 100 000, P < 0.001).
The incidence of IPD caused by 7vPCV serotypes in 2007 was comparable between Indigenous and non-Indigenous children (3.7 v 2.1 per 100 000, P = 0.56). However, the incidence of IPD caused by non-7vPCV serotypes remained significantly higher among Indigenous children compared with non-Indigenous children (78.6 v 15.7 per 100 000, P < 0.001).
Box 3 shows the change in IPD incidence among non-Indigenous children aged < 2 years after the introduction of the universal childhood 7vPCV immunisation program in 2005. Compared with the mean incidence of IPD between 2002 and 2004 (77.7 per 100 000), the overall incidence decreased by 75% in 2006 and 2007 (to 19.6 and 19.5 per 100 000, respectively, P < 0.001). The incidence of IPD caused by 7vPCV serotypes decreased by 91% in 2006 (5.3 per 100 000, P < 0.001) and 97% in 2007 (2.1 per 100 000, P < 0.001), compared with 2002–2004 (60.9 per 100 000).
Among the non-7vPCV serotypes, the incidence of IPD caused by serotype 19A showed a significant increase of 110% in 2006 (5.7 per 100 000, P < 0.01) and 215% in 2007 (8.6 per 100 000, P < 0.001), compared with 2002–2004 (2.7 per 100 000), to become the largest cause of IPD. There were also significant increases in 2007 in the incidence of cases caused by serotypes 11A (from 0.1 to 1.2 per 100 000, P < 0.05) and 22F (from 0.3 to 1.3 per 100 000, P < 0.05). Although there were some changes in the incidence of IPD caused by serotypes 6A and 3 in 2006, these marginally significant differences were not maintained in 2007.
Box 4 shows the cumulative proportion of IPD cases in 2007 in all Australian children aged < 2 years caused by serotypes covered by 7vPCV, as well as additional serotypes included in the 10-valent and 13-valent pneumococcal conjugate vaccines (10vPCV and 13vPCV). These included serotypes 1, 5 and 7F (covered by both 10vPCV and 13vPCV) and serotypes 3, 6A and 19A (covered by 13vPCV only). Compared with 7vPCV, the six additional serotypes covered in 13vPCV accounted for an additional 50% of the IPD cases. Serotype 19A caused the highest proportion of IPD cases in 2007 (37.7%).
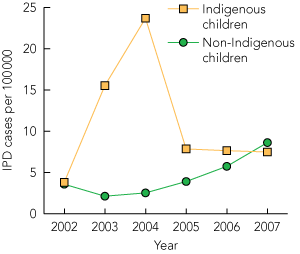
The change in incidence of IPD caused by serotype 19A in children aged < 2 years between 2002 and 2007 is illustrated in Box 5. There was no significant increase in the incidence of IPD caused by serotype 19A among Indigenous children (P = 0.71). In contrast, the incidence of serotype 19A IPD showed a significant increase among non-Indigenous children (P < 0.001).
Since the inclusion of 7vPCV in the National Immunisation Program in Australia, the incidence of IPD has declined dramatically. Between 2002 and 2007, the incidence of IPD decreased by 74% among all Australian children under the age of 2 years. The decline was observed to occur later among non-Indigenous children (2005 onwards) than Indigenous children (2002 onwards), reflecting the earlier introduction of 7vPCV vaccination for Indigenous children compared with non-Indigenous children (mid 2001 v 2005). This decrease in IPD incidence was predominantly driven by a large reduction in IPD cases caused by 7vPCV serotypes.
Several studies have shown an increase in IPD caused by non-7vPCV serotypes following the introduction of 7vPCV vaccination.7,9,10,15 Similarly, in our study, the overall decrease in the incidence of IPD was partly offset by a significant increase in the incidence of IPD caused by non-7vPCV serotypes in non-Indigenous children. Serotype-level analyses showed that this increase was largely attributable to an increase in serotype 19A. An increase in serotype 19A has been previously reported in other countries, such as the US, Canada and Spain. This is of particular concern, as serotype 19A has been found to exhibit multidrug resistance14,15,20,21 and may exhibit greater potential for invasiveness.22 We found that serotype 19A accounted for 38% of IPD cases among Australian children under the age of 2 years in 2007. As such, the inclusion of serotype 19A in a pneumococcal conjugate vaccine for children would provide significantly increased coverage for IPD in the National Immunisation Program.
In contrast, no significant increase in serotype 19A or non-7vPCV serotypes as a group was observed among Indigenous children. In spite of this, the incidence of non-7vPCV serotype IPD remained significantly higher in this group than in non-Indigenous children. The reasons for this difference are likely to be multifactorial. A North American study that examined risk factors for IPD in children found that ethnicity was not an independent risk factor when other factors such as underlying diseases and lack of breastfeeding were taken into account.23 This suggests that differences between Indigenous and non-Indigenous children may be a result of differential rates of independent risk factors. It is also important to note that the smaller population of Indigenous children in our study and smaller absolute number of cases among them render the data more susceptible to random fluctuations and may have reduced statistical power to detect trends. Nonetheless, the use of a conjugate vaccine with a broader range of serotypes, together with efforts to reduce modifiable risk factors, may be particularly beneficial for Indigenous children.
While some studies have suggested that the increase in serotype 19A may be a result of serotype replacement after the introduction of 7vPCV,7,10 a study in South Korea reported that serotype 19A increased even before the introduction of 7vPCV, and showed that the expansion of a multidrug-resistant clone (ST320) was responsible for the increase.24 Others have suggested that the emergence of serotype 19A in various populations is likely due to multiple factors, including the baseline prevalences of 19A and particular virulent clones, antibiotic use patterns, and frequency of pneumococcal transmission.25,26
The incidence of several other serotypes also showed an apparent change in non-Indigenous children after the introduction of 7vPCV vaccination. A decrease in IPD incidence caused by serotype 6A that was observed in 2006 compared with the pre-vaccination period may be a result of limited cross-protection afforded by serotype 6B in 7vPCV, as this has been shown to be cross-reactive with serotype 6A.27 However, this decrease was not sustained in 2007. The extent of cross-protection for serotype 6A provided by a three-dose schedule, as used in Australia (3+0), is not well studied, and identifying the contribution of the recently identified serotype 6C to these data would also be important. Serotypes 11A and 22F showed an apparent significant increase in 2007; however, owing to the small number of cases (< 10), and taking into account the multiple comparisons performed in this study, we are cautious about interpreting these results. Interestingly, no significant change in the incidence of IPD caused by serotypes 1 and 5, known to be responsible for epidemic outbreaks of IPD,28 was observed. In fact, there was a single case of serotype 1 IPD and no cases of serotype 5 IPD reported during the study period. Overall, these results highlight the importance of monitoring future trends at the serotype level.
A limitation of our study is the lack of data on the vaccination status of children with IPD, which means no observations on vaccine failure can be made. Another caveat is the lack of information on independent risk factors for IPD. Inclusion of such information would provide a clearer indication of the differences, if any, between Indigenous and non-Indigenous children. Nonetheless, such factors are unlikely to have changed drastically over the relatively short study period, and the trends observed within each group should remain valid.
While there is evidence of the emergence of non-7vPCV serotypes, particularly 19A, the effect of 7vPCV in reducing IPD incidence among both Indigenous and non-Indigenous children in Australia remains significant. The addition of serotype 19A to conjugate vaccines that cover a wider range of serotypes would further improve the impact of the immunisation program in reducing the incidence of IPD.
1 Incidence of invasive pneumococcal disease (IPD) in Australian children < 2 years of age, by Indigenous status, 2002–2007
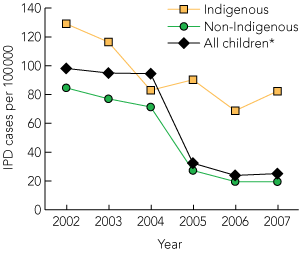 * Includes children of unknown Indigenous status. |
2 Incidence of 7-valent pneumococcal conjugate vaccine (7vPCV) serotype and non-7vPCV serotype invasive pneumococcal disease (IPD) in children aged < 2 years, 2002–2007
3 Change in invasive pneumococcal disease incidence in non-Indigenous children < 2 years of age after the introduction of the 7-valent pneumococcal conjugate vaccine (7vPCV) immunisation program in 2005*
|
Before 7vPCV program |
After 7vPCV program |
|||||||||||||
|
2002–2004 |
2006 |
2007 |
||||||||||||
|
No. of cases |
Mean cases/year |
Mean incidence |
No. of cases |
Incidence |
Change from |
No. of cases |
Incidence |
Change from |
||||||
7vPCV serotypes |
|
|
|
|
|
|
|
|
|||||||
4 |
56 |
18.7 |
3.9 |
3 |
0.6 |
− 84.9%‡ |
1 |
0.2 |
− 95.1%‡ |
||||||
6B |
160 |
53.3 |
11.2 |
4 |
0.8 |
− 93.0%‡ |
2 |
0.4 |
− 96.6%‡ |
||||||
9V |
43 |
14.3 |
3.0 |
0 |
0 |
na |
1 |
0.2 |
− 93.7%‡ |
||||||
14 |
380 |
126.7 |
26.7 |
3 |
0.6 |
− 97.8%‡ |
1 |
0.2 |
− 99.3%‡ |
||||||
18C |
54 |
18.0 |
3.8 |
6 |
1.2 |
− 68.7%§ |
1 |
0.2 |
− 94.9%‡ |
||||||
19F |
122 |
40.7 |
8.6 |
7 |
1.4 |
− 83.8%‡ |
4 |
0.8 |
− 91.1%‡ |
||||||
23F |
53 |
17.7 |
3.7 |
4 |
0.8 |
− 78.7%¶ |
1 |
0.2 |
− 94.9%‡ |
||||||
Subtotal |
868 |
289.3 |
60.9 |
27 |
5.3 |
− 91.2%‡ |
11 |
2.1 |
− 96.5%‡ |
||||||
Non-7vPCV serotypes |
|
|
|
|
|
|
|
||||||||
1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0.2 |
na |
||||||
5 |
0 |
0 |
0 |
0 |
0 |
na |
0 |
0 |
na |
||||||
7F |
6 |
2.0 |
0.4 |
3 |
0.6 |
40.9% |
1 |
0.2 |
− 54.5% |
||||||
3 |
8 |
2.7 |
0.6 |
8 |
1.6 |
182%¶ |
1 |
0.2 |
− 65.9% |
||||||
6A |
30 |
10.0 |
2.1 |
3 |
0.6 |
− 71.8%¶ |
6 |
1.1 |
− 45.4% |
||||||
19A |
39 |
13.0 |
2.7 |
29 |
5.7 |
110%§ |
45 |
8.6 |
215%‡ |
||||||
11A |
2 |
0.7 |
0.1 |
0 |
0 |
na |
6 |
1.2 |
819%¶ |
||||||
22F |
4 |
1.3 |
0.3 |
2 |
0.4 |
141% |
7 |
1.3 |
478%¶ |
||||||
33F |
3 |
1.0 |
0.2 |
0 |
0 |
na |
3 |
0.6 |
273% |
||||||
Others** |
28 |
9.3 |
2.0 |
16 |
3.2 |
161% |
12 |
2.3 |
117% |
||||||
Subtotal |
120 |
40.0 |
8.4 |
61 |
12.1 |
143%¶ |
82 |
15.7 |
186%‡ |
||||||
Untyped†† |
119 |
39.7 |
8.4 |
11 |
2.2 |
− 74.0%‡ |
9 |
1.7 |
− 79.4%‡ |
||||||
Total |
1107 |
369.0 |
77.7 |
99 |
19.6 |
− 74.8%‡ |
102 |
19.5 |
− 74.9%‡ |
||||||
na = not applicable. * Cases during the first year of the 7vPCV immunisation program (2005) were excluded from analyses to allow time for vaccine uptake. † Significance testing performed using the Fisher exact test, to compare the change in incidence between baseline (2002–2004) and 2006 and 2007. ‡ P < 0.001. § P < 0.01. ¶ P < 0.05. ** “Others” group includes serotypes 7C, 8, 10A, 12F, 15B, 15C, 17F, 18A, 23A, 24F, 35F and 35B. †† Untyped group comprises cases where the isolates were not serotyped. |
|||||||||||||||
4 Cumulative proportion of cases of invasive pneumococcal disease (IPD) caused by various serotypes, among all children aged < 2 years* in 2007
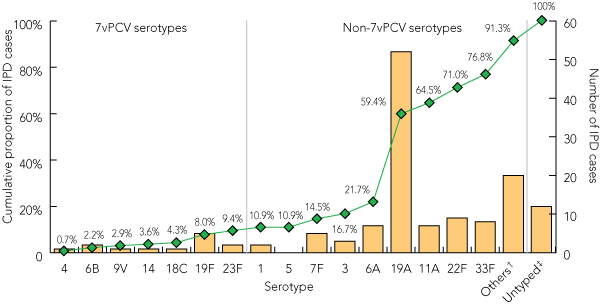 7vPCV = 7-valent pneumococcal conjugate vaccine. Line graph shows the cumulative proportion of IPD cases caused by 7vPCV and selected non-7vPCV serotypes (including serotypes 1, 5 and 7F, which are covered by both 10vPCV and 13vPCV; and serotypes 3, 6A and 19A, which are covered by 13vPCV only). Bars depict number of IPD cases caused by each serotype. * Includes children of unknown Indigenous status. † “Others” group includes serotypes 7C, 8, 10A, 12F, 15B, 15C, 17F, 18A, 23A, 24F, 35F and 35B. ‡ Untyped group comprises cases where the isolates were not serotyped. |
Received 23 June 2010, accepted 24 October 2010
- Scott R Williams1
- Paul J Mernagh2
- Michael H T Lee1
- Jonathan T Tan2
- 1 Specialty Care Business Unit, Pfizer Australia, Sydney, NSW.
- 2 Health Technology Analysts, Sydney, NSW.
We gratefully acknowledge the Communicable Diseases Network Australia for providing the NNDSS data used in this study, the Office of Health Protection of the Australian Government Department of Health and Ageing, the Pneumococcal Working Party of the Communicable Diseases Network Australia, the Enhanced Invasive Pneumococcal Disease Surveillance Working Group and the individual state and territory laboratories for their support of laboratory surveillance of IPD and collection of data.
Scott Williams and Michael Lee are employees of Pfizer Australia, hold shares in Pfizer and are former employees of Wyeth Australia. Wyeth is the sponsor of 7vPCV (Prevenar) and 13vPCV (Prevenar 13). Wyeth is part of the Pfizer global group of companies. Pfizer supports the authorship criteria established by the International Committee of Medical Journal Editors. Paul Mernagh and Jonathan Tan are employees of Health Technology Analysts, which received consulting fees from Wyeth for their contribution to writing and reviewing the manuscript and the analysis and reporting of results.
- 1. O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374: 893-902.
- 2. Hsu HE, Shutt KA, Moore MR, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med 2009; 360: 244-256.
- 3. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348: 1737-1746.
- 4. Tyrrell GJ, Lovgren M, Chui N, et al. Serotypes and antimicrobial susceptibilities of invasive Streptococcus pneumoniae pre- and post-seven valent pneumococcal conjugate vaccine introduction in Alberta, Canada, 2000–2006. Vaccine 2009; 27: 3553-3560.
- 5. Roche PW, Krause V, Cook H, et al. Invasive pneumococcal disease in Australia, 2006. Commun Dis Intell 2008; 32: 18-30.
- 6. Watson M, Roche P, Bayley K, et al. Laboratory surveillance of invasive pneumococcal disease in Australia, 2003 predicting the future impact of the universal childhood conjugate vaccine program. Commun Dis Intell 2004; 28: 455-464.
- 7. Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska Native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 2007; 297: 1784-1792.
- 8. Bettinger JA, Scheifele DW, Kellner JD, et al. The effect of routine vaccination on invasive pneumococcal infections in Canadian children, Immunization Monitoring Program, Active 2000–2007. Vaccine 2010; 28: 2130-2136.
- 9. Salleras L, Dominguez A, Ciruela P, et al. Changes in serotypes causing invasive pneumococcal disease (2005–2007 vs. 1997–1999) in children under 2 years of age in a population with intermediate coverage of the 7-valent pneumococcal conjugated vaccine. Clin Microbiol Infect 2009; 15: 997-1001.
- 10. Guevara M, Barricarte A, Gil-Setas A, et al. Changing epidemiology of invasive pneumococcal disease following increased coverage with the heptavalent conjugate vaccine in Navarre, Spain. Clin Microbiol Infect 2009; 15: 1013-1019.
- 11. Lacapa R, Bliss SJ, Larzelere-Hinton F, et al. Changing epidemiology of invasive pneumococcal disease among White Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin Infect Dis 2008; 47: 476-484.
- 12. Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA 2006; 295: 1668-1674.
- 13. Vestrheim DF, Lovoll O, Aaberge IS, et al. Effectiveness of a 2+1 dose schedule pneumococcal conjugate vaccination programme on invasive pneumococcal disease among children in Norway. Vaccine 2008; 26: 3277-3281.
- 14. Dagan R. Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Infect 2009; 15 Suppl 3: 16-20.
- 15. Pai R, Moore MR, Pilishvili T, et al. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J Infect Dis 2005; 192: 1988-1995.
- 16. Giele CM, Keil AD, Lehmann D, Van Buynder PG. Invasive pneumococcal disease in Western Australia: emergence of serotype 19A [letter]. Med J Aust 2009; 190: 166. <MJA full text>
- 17. Hanna JN, Humphreys JL, Murphy DM. Invasive pneumococcal disease in Indigenous people in north Queensland: an update, 2005–2007. Med J Aust 2008; 189: 43-46. <MJA full text>
- 18. Australian Bureau of Statistics. Population by age and sex, Australian states and territories, Jun 2008 (updated Mar 2009). Canberra: ABS, 2009. (ABS Cat. No. 3201.0.)
- 19. Australian Bureau of Statistics. Experimental estimates and projections, Aboriginal and Torres Strait Islander Australians, 1991 to 2021, Series B. Canberra: ABS, 2009. (ABS Cat. No. 3238.0.)
- 20. Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis 2007; 196: 1346-1354.
- 21. Jacobs MR, Good CE, Beall B, et al. Changes in serotypes and antimicrobial susceptibility of invasive Streptococcus pneumoniae strains in Cleveland: a quarter century of experience. J Clin Microbiol 2008; 46: 982-990.
- 22. Hanage WP, Kaijalainen TH, Syrjanen RK, et al. Invasiveness of serotypes and clones of Streptococcus pneumoniae among children in Finland. Infect Immun 2005; 73: 431-435.
- 23. Levine OS, Farley M, Harrison LH, et al. Risk factors for invasive pneumococcal disease in children: a population-based case-control study in North America. Pediatrics 1999; 103: E28.
- 24. Choi EH, Kim SH, Eun BW, et al. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis 2008; 14: 275-281.
- 25. Dagan R, Givon-Lavi N, Leibovitz E, et al. Introduction and proliferation of multidrug-resistant Streptococcus pneumoniae serotype 19A clones that cause acute otitis media in an unvaccinated population. J Infect Dis 2009; 199: 776-785.
- 26. Moore MR, Gertz RE Jr, Woodbury RL, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis 2008; 197: 1016-1027.
- 27. Vakevainen M, Eklund C, Eskola J, Kayhty H. Cross-reactivity of antibodies to type 6B and 6A polysaccharides of Streptococcus pneumoniae, evoked by pneumococcal conjugate vaccines, in infants. J Infect Dis 2001; 184: 789-793.
- 28. Torzillo PJ, Morey F, Gratten M, et al. Changing epidemiology of invasive pneumococcal disease in central Australia prior to conjugate vaccine: a 16-year study. Vaccine 2007; 25: 2375-2378.





Abstract
Objective: To evaluate trends in the incidence and serotype profile of invasive pneumococcal disease (IPD) in Australian children under 2 years of age after the introduction of the 7-valent pneumococcal conjugate vaccine (7vPCV).
Design and setting: Analysis of incidence rates calculated using IPD surveillance data (including age, Indigenous status and serotype of the pneumococcal isolate) from 2002 to 2007 obtained from the National Notifiable Diseases Surveillance System and population estimates obtained from the Australian Bureau of Statistics.
Main outcome measures: Trends in IPD incidence among Indigenous and non-Indigenous children between 2002 and 2007; change in the serotype profile of IPD in non-Indigenous children after the introduction of universal 7vPCV vaccination in 2005.
Results: Overall incidence of IPD decreased by 74% in all children < 2 years of age between 2002 and 2007 (P < 0.001). While the incidence of IPD caused by 7vPCV serotypes decreased significantly among both Indigenous and non-Indigenous children, the incidence of non-7vPCV serotype IPD increased significantly in non-Indigenous children (from 9.7 to 15.7 per 100 000, P < 0.001). Compared with a pre-vaccination period (2002–2004), the 2007 incidence of serotype 19A IPD in non-Indigenous children increased significantly (from 2.7 to 8.6 per 100 000, P < 0.001). In 2007, 19A was the predominant serotype causing IPD (37.7%) in all children aged < 2 years.
Conclusions: The overall incidence of IPD decreased from 2002 to 2007, primarily driven by a reduction in IPD caused by 7vPCV serotypes. However, this was partially offset by a significant increase in the incidence of IPD caused by non-7vPCV serotypes, particularly 19A, in non-Indigenous children.