Herpes zoster is associated with several neurological complications, including postherpetic neuralgia, aseptic meningitis, meningoencephalitis, transverse myelitis, peripheral nerve palsies, cranial nerve palsies and granulomatous cerebral angiitis.1 These complications are particularly prevalent among older people and patients with immunodeficiency. A causative relationship with herpes zoster in many of these syndromes is probably more common than suspected owing to difficulties in diagnosis and lack of awareness among clinicians. However, to our knowledge, there are no published reports of any known association between the neurological complications of herpes zoster, including myelitis, and postherpetic neuralgia.
Transverse myelitis is a focal inflammatory disorder of the spinal cord. It results in sensory, motor and autonomic dysfunction 2 Onset may be acute, developing over a few hours or several days, or subacute, developing over 1 to 2 weeks. The critical factor is an abnormal immune response to infection, rather than the direct effect of an infectious agent.3 Transverse myelitis may be an isolated entity or may occur with a background of viral diseases, vaccinations, systemic lupus erythematosus, vasculitis, multiple sclerosis, heroin misuse or trauma.3 About 25%–40% of cases of transverse myelitis are caused by viral infections with herpes viruses or poliovirus.2 A recent publication provides useful additional information about transverse myelitis.4
Lessons from practice
Herpes zoster myelitis is rare in the context of normal immunity; it is more common among patients with immunocompromise.
Clinicians should be aware of the close temporal relationship between skin rash and the onset of myelitis so that appropriate investigations and treatment can be instigated.
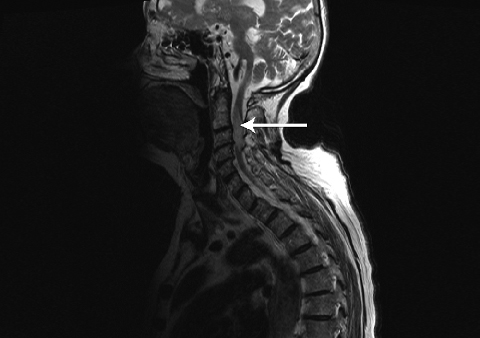
Magnetic resonance imaging of the spine should be performed to aid diagnosis.
Although early treatment of herpes zoster myelitis is preferable, delayed treatment may also be worthwhile.
Transverse myelitis following herpes zoster or herpes zoster myelitis (HZM) is rare, and typically occurs in hosts who are immunocompromised. Its onset is usually acute, occurring shortly after the appearance of the rash, with the development of sensory, motor and autonomic dysfunction.5 No diagnostic test is completely accurate, as the virus cannot usually be isolated from blood or cerebrospinal fluid in HZM.5 In most cases, diagnosis of HZM is clinical and based on detection of typical vesicular lesions in dermatomal distribution in association with clinical features of transverse myelitis. Suggested treatment involves high doses of corticosteroids and aciclovir.5 The prognosis ranges from spontaneous recovery to ascending neurological progression and death.6
The frequency of transverse myelitis during or after varicella infection is reported to be 0.3%.7 Devinsky and colleagues analysed their findings in 13 immunocompromised patients with HZM.8 The pathogenesis of HZM is unclear, but it may be due to direct viral invasion, which was demonstrated in one autopsy case.9
Diagnosing HZM can be challenging. The importance of a careful clinical assessment to establish the likelihood of this diagnosis and the level of the spinal cord damage, in combination with confirmatory magnetic resonance imaging (MRI)7cannot be overstated. MRI not only provides information about the site but also the extent of spinal cord involvement, and excludes other possible diagnoses. In our patient, HZM was diagnosed based on the temporal relationship of myelopathy to the rash and MRI findings. Although the area of the spinal cord that was involved on the MRI scan was less extensive than that affected by the herpetic rash, we do not see this as clinically inconsistent.
There are no proven treatment regimens for HZM, but there is anecdotal evidence for treatment of HZM with high doses of aciclovir and corticosteroids.5,10 It appears that antiviral treatment was not provided early to our patient because it was not initially recognised that the rash was due to herpes zoster. Once HZM was diagnosed, this treatment was provided. Despite the delay of 3 weeks following the onset of rash, improvement appeared consistent with a clinical response to this therapy. Although early treatment of herpes zoster with antivirals is crucial to prevent the development of postherpetic neuralgia, there is little evidence that such treatment reduces the risk of HZM.
To date, no evidence has emerged regarding the protective efficacy of the new live, attenuated herpes zoster vaccine (Zostavax) against HZM. Trials assessing the impact of antivirals or the herpes zoster vaccine on risk of HZM would require very large numbers of participants, given the rarity of this complication. Nevertheless, the vaccine has proven to be efficacious in reducing the incidence of and morbidity associated with herpes zoster and postherpetic neuralgia in older adults.11 It has been noted in a previous case report that, following the diagnosis of HZM, even delayed treatment with oral antivirals may prevent neurological progression.12 Our case provides support for this assertion.
- 1. Antonio V. Severe complications of herpes zoster. Herpes 2007; 14 Suppl 2: 35A-39A.
- 2. Marra C. Infections associated with myelopathy, radiculopathy and neuropathy. In: Root RK, Waldvogel F, Corey L, Stamm WE, editors. Clinical infectious diseases: a practical approach. New York: Oxford University Press, 1999: 715-722.
- 3. Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002; 59: 499-505.
- 4. Frohman EM, Wingerchuk DM. Transverse myelitis. N Engl J Med 2010; 363: 564-572.
- 5. Yÿlamz S, Köseolu HK, Yücel E. Transverse myelitis caused by varicella zoster: case reports. Braz J Infect Dis 2007; 11: 179-181.
- 6. Jubelt B. Viral infections and postviral syndromes. In: Rowland LP, Pedley TA, editors. Merritt’s neurology. 12th ed. Philadelphia: Lippincott Williams & Wilkins, 2009: 156-185.
- 7. Hyrai T, Korogi Y, Hamatake S. Case report: varicella virus myelitis — serial MR findings. Br J Radiol 1996; 69: 1187-1190.
- 8. Devinsky O, Cho ES, Petiko CK. Herpes zoster myelitis. Brain 1991; 114: 1181-1196.
- 9. Hogan EL, Krigman MR. Herpes zoster myelitis. Evidence for viral invasion of spinal cord. Arch Neurol 1973; 29: 309-313.
- 10. Outteryck O, Deramecourt V, Bombois S, et al. VZV-related myelitis: a pathophysiological hypothesis. Rev Neurol 2007; 163: 89-92.
- 11. Anthony LC, Judith B, Dominic ED, David WG. The prevention and management of herpes zoster. Med J Aust 2008; 188: 171-176. <MJA full text>
- 12. de Silva SM, Mark AS, Gilden DH, et al. Zoster myelitis: improvement with antiviral therapy in two cases. Neurology 1996; 47: 929-9231.





None identified.