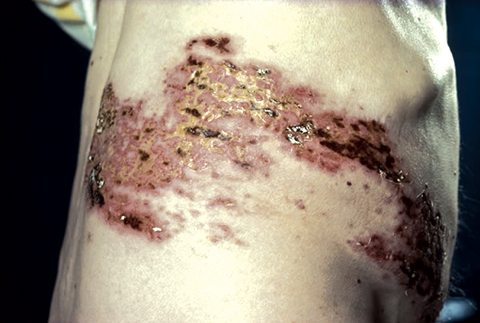
Herpes zoster (HZ) is characterised by a unilateral, painful, vesicular rash with a dermatomal distribution. It is a manifestation of the reactivation of latent varicella-zoster virus (VZV), which, as a primary infection, produces chickenpox (varicella).1 Although the rash is its most distinctive feature, HZ is frequently not a benign disease; zoster-associated pain, both acute and chronic, can be debilitating and poorly responsive to treatment once established.1-3 The proven effectiveness of a live, attenuated VZV vaccine (Oka/Merck strain, Zostavax [Merck Sharp & Dohme, distributed in Australia by CSL Limited; trade name used for product identification only, and no endorsement is implied]) in reducing the incidence of and morbidity associated with HZ and postherpetic neuralgia (PHN) is a major advance.3 Here, we provide an overview of the new vaccine and introduce clinicians to a new clinical paradigm — the prevention of HZ. We also apply current opinion and evidence to the management of HZ.
Results from a national serosurvey show that most Australians are infected with VZV and are therefore at risk of HZ.4 Estimates from extrapolated data suggest that about 150 000 cases of HZ occur within the Australian community each year — a rate of 830 per 100 000 population.5 The Australian General Practice Research Network (GPRN) database reports that between January 2001 and December 2005, 10% of patients with HZ developed PHN, and the risk increased with age. The annual direct health care cost to Australia during this period was estimated to be $19–$30 million (Lynne Conway, Director, Health Economics, CSL Limited, personal communication). There is a direct correlation between the incidence of HZ and increasing age,6-8 so these figures are expected to increase with population ageing. Universal varicella vaccination, funded by the National Immunisation Program since 2005, may also alter the future epidemiology of HZ and its sequelae; rates of HZ may increase because of reduced exposure to circulating wild-type VZV.5,8
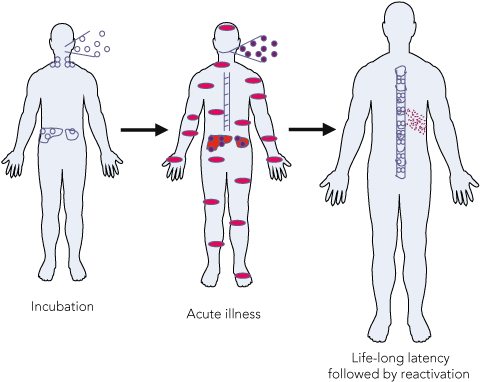
One of eight human herpes viruses, VZV infects 90% of the population by adulthood, establishing latency within the dorsal root ganglia (see Box 1).1,9-11 Cell-mediated immunity is now known to be more important than humoral immunity for protection against VZV infection, although VZV-specific antibody levels can be used as an index of vaccine efficacy (positive titres 4–6 weeks after vaccination indicate protection).10 Exogenous, circulating wild-type virus episodically boosts adult T-cell immunity (eg, exposure to infected children) so that reactivation usually occurs as a result of naturally waning cell-mediated immunity with age or induced immunosuppression.10,11 PHN is currently thought to be the result of neuronal damage and scarring to the affected dorsal root ganglion after the extensive inflammation of HZ.2,11,12
A prodrome characterised by pain and other localised skin sensations (eg, burning, tingling and itching), headache, photophobia, malaise and (rarely) fever typically precedes the appearance of the zoster rash by 2–3 days (Box 2). The rash, often pruritic, spreads throughout the affected dermatome, evolving through papular, vesicular (3–5 days) and crusting (7–10 days) stages, taking 2–4 weeks to heal.1,2,9,11
Acute pain accompanies the rash in 80% of individuals aged over 50 years, varying in both nature (burning to lancinating) and severity.2,11 It may be accompanied by parasthesiae, anaesthesia or allodynia (pain induced by touch, often from trivial stimuli such as clothing).9,11 Although usually self-limiting, pain may persist beyond the resolution of the rash — PHN, defined as pain persisting for 90 or more days after the onset of pain or rash, can last months and even years.2,9
Zoster ophthalmicus (ranging from keratitis to the more severe iritis) is a relatively common complication of HZ, affecting 10%–20% of patients.2,9,11 Rarer complications include motor neurone and peripheral nerve palsies (eg, Bell’s palsy and Ramsay Hunt syndrome), as well as meningitis, myelitis, vasculitis, paresis and urinary retention owing to visceral involvement.2,9,11
Herpes zoster is usually diagnosed clinically. Laboratory diagnosis (involving the detection of VZV antigens or nucleic acid, or isolation of virus, from swabs of lesions, or by VZV-specific IgM antibody tests) is recommended when the clinical picture is atypical or complicated (eg, when there is persistent or recurrent rash, a single lesion, central nervous system involvement or immunosuppression).11,13
Antiviral therapy for HZ is currently underprescribed in Australia. Data from the Restricted Pharmaceuticals Benefits Scheme for the period 1995–1999 indicate that only 40% of all community cases of HZ were treated with antiviral drugs.5 The proportion of this trend resulting from late presentations (beyond 72 hours) versus non-prescribing is unclear. The GPRN database recorded an antiviral drug treatment rate of 61% for 2001–2005, suggesting a trend towards increased prescribing of antiviral medications (Lynne Conway, Director, Health Economics, CSL Limited, personal communication).
Aciclovir, valaciclovir and famciclovir have proven efficacy in managing acute HZ, accelerating the resolution of acute pain and skin lesions, while reducing the duration and possibly the incidence of PHN.14-16 Famciclovir and valaciclovir have replaced aciclovir as the drug of choice because of their more favourable pharmacokinetic profiles and simpler dosing regimens.2
As antiviral drugs are most effective when started within 72 hours of rash onset, early presentation should be encouraged.17 However, existing data suggest benefit might extend beyond this time period, and antiviral therapy initiated at or shortly after 72 hours should be considered if pain is severe or lesions are progressive.2,9
Antiviral therapy is particularly indicated if the patient is aged over 50, is immunocompromised, has ophthalmic zoster, or if PHN is more likely (Box 3).11 The favourable risk–benefit profile of antiviral medications, however, means antiviral therapy should be considered even for patients deemed at low risk of developing PHN and other complications.9
Therapy with oral corticosteroids (eg, 40 mg prednisolone daily for 7 days, tapering to 5 mg daily over the next 2 weeks) can reduce symptoms in the acute (inflammatory) phase of HZ, but only when used in combination with an antiviral agent.18,19 Corticosteroids are recommended for patients over the age of 50 if not contraindicated,20 and should be used with caution in patients with comorbid conditions such as diabetes.2,9,20 Corticosteroids are not effective in preventing or managing PHN.18-20
Zoster-associated pain should be treated early and aggressively, as evidence suggests it is more difficult to treat once established.2,9,11 As necessary, treatment should ascend the “analgesic ladder” from paracetamol/non-steroidal anti-inflammatory drugs to codeine, morphine or oxycodone.2 The need for analgesia frequently escalates during the acute period, so regular follow-up (every 2–3 days) is recommended until pain is controlled. Box 4 provides a guide for assessing acute pain severity and the presence of neurological dysfunction.
Patients at high risk for developing PHN (see Box 3) should receive proactive follow-up to enable the prompt diagnosis of PHN following HZ. However, there is little consensus within Australia, or overseas, about how best to manage the pain of PHN. We present one suggested algorithm in Box 5. A case vignette illustrating the high psychosocial impact of PHN is presented in Box 6.
In the United States Shingles Prevention Study (SPS), a double-blind, placebo-controlled, multicentre trial involving 38 546 participants aged 60 years or over, a live, attenuated, Oka/Merck strain, VZV vaccine (containing a viral titre 14 times higher than the current varicella vaccine) significantly altered the natural history of latent VZV, preventing HZ in the “young” old (60–69 years) and either preventing or attenuating HZ in older participants.3 Overall, the vaccine reduced the burden of illness from HZ by 61.1% (P < 0.001) and reduced its incidence by 51.3% (P < 0.001). The duration of pain and discomfort among participants with confirmed HZ was significantly shorter in the vaccine group (21 days v 24 days; P = 0.03), and the incidence of PHN was reduced by 66.5% (P < 0.001).3
The vaccine (Zostavax) is currently indicated in Australia for the prevention of HZ in individuals aged 50 years and older, and for the prevention of HZ and PHN and reduction of acute and chronic zoster-associated pain in individuals aged 60 years and older. An application to the federal government for the vaccine to be listed on the publicly funded National Immunisation Program is pending. As the only vaccine able to prevent the clinical recurrence of a pre-existing latent viral infection (and which does not induce herd immunity), Zostavax represents a unique case for National Immunisation Program funding. The availability of this vaccine is stimulating more accurate studies of the health care costs of zoster and PHN, and vaccine cost-effectiveness analyses, especially to help appraise the case for national funding.3,6 These should be discussed in depth in future publications when key data are available.
Worldwide epidemiological data suggest that the ideal age for HZ vaccination would probably be before 50–55 years, the time at which the incidence of HZ begins to increase exponentially.6,7,8 From a biological viewpoint, it is likely that a younger individual would mount a greater immune response to the vaccine. This supports vaccinating individuals 5–10 years earlier than currently indicated (ie, at 50–59 years). Precautions or contraindications to the vaccine are also likely to be less troublesome in a slightly younger age group. The potential loss of environmental boosting of VZV cell-mediated immunity after the introduction of universal varicella vaccination may mean that the age-specific incidence curve for HZ shifts to a younger age group.5 This epidemiological trend, should it eventuate, would also provide a strong case for vaccinating people at a younger age.
It is currently recommended that the zoster vaccine be given as a single dose.3 Trial data indicate that the vaccine’s efficacy will persist for at least 4 years, with plateauing at about 1 year.3 Without any evidence of waning immunity, we expect the vaccine’s efficacy to endure much longer than this. The ongoing surveillance of SPS-trial participants will show the total duration of protection that the vaccine affords and whether a booster dose is required.3
In general, live vaccines are safe when administered before or after an inactivated vaccine.23 In a double-blind, placebo-controlled trial of 762 participants aged 50 years or older, the zoster vaccine induced a similar antibody response after 4 weeks when administered concomitantly or non-concomitantly with the influenza vaccine.24 No safety data exist on the concomitant administration of the zoster vaccine with the pneumococcal (polysaccharide) or diphtheria–tetanus–acellular pertussis vaccines.
Live vaccines are usually contraindicated in immunosuppressed people. Clinicians should refer to the Australian immunisation handbook for guidelines on administering live virus vaccines to this patient group.23 In patients for whom HZ vaccination is contraindicated, the priority should be early diagnosis of HZ and prompt treatment with antiviral agents. The effectiveness of a “killed” vaccine for use in immunosuppressed individuals is currently being explored.
The epidemiology of HZ in Australia is well described; clinicians can assume that any long-term resident of Australia is VZV immune. The immune status of foreign-born individuals cannot be assumed; VZV exposure has been reported to be as low as 40%–50% in some tropical countries.25 However, the zoster vaccine was safe when given to 125 subjects who had never had primary varicella infection.26
The use and safety of vaccinating an individual who has had HZ in the distant past (estimated to be about 15% of the target population) is currently being studied. The time interval is likely to be important; the rationale behind vaccinating a 70-year-old following an episode of HZ at age 63 is not compelling, but a strong argument could be made for vaccinating the same individual if the HZ occurred at the age of 40. The US Advisory Committee on Immunization Practices currently recommends vaccination regardless of prior history of HZ.27
1 Pathogenesis of varicella-zoster virus
3 Predicting risk of postherpetic neuralgia (PHN)2,9,11
The three main risk factors that predict PHN following herpes zoster are:
The risk of PHN will rise as the number of risk factors present increases.
4 Guide to assessing severity of acute zoster pain and neurological dysfunction
5 An algorithm for the pharmacological management of established postherpetic neuralgia (PHN)*†
- Anthony L Cunningham1
- Judith Breuer2
- Dominic E Dwyer1
- David W Gronow3
- Robert D Helme4
- John C Litt5
- Myron J Levin6
- C Raina MacIntyre7
- 1 Westmead Millennium Institute for Medical Research and University of Sydney, Sydney, NSW.
- 2 Centre for Infectious Diseases, Barts and The London School of Medicine and Dentistry, London, UK.
- 3 Pain Services Westmead Hospital and Sydney Pain Management Centre, Sydney, NSW.
- 4 Western Health, Melbourne, VIC.
- 5 Department of General Practice, Flinders University, Adelaide, SA.
- 6 University of Colorado, Boulder, Colo, USA.
- 7 National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases, The Children’s Hospital at Westmead, Sydney, NSW.
The article was developed with an unrestricted educational grant from CSL Limited. The company did not participate in any author meetings nor exert any editorial influence. We thank Dr Robert W Johnson and Professor Margaret Burgess for their review of the draft manuscript, and Wellmark and Dr Candice O’Sullivan for their help in preparing the manuscript.
The authors were paid an honorarium for their attendance and participation at the roundtable meeting, and their review of the draft manuscript. Travel support was also provided if required. All members of the roundtable group except Myron Levin and Judith Breuer are members of the Zostavax Advisory Board, convened on a regular basis by CSL Limited. Robert Helme is a member of the Neuropathic Pain Lyrica Advisory Board (Pfizer). Raina MacIntyre is a member of the VZV Working Party of the Australian Technical Advisory Group on Immunisation. Myron Levin has received honoraria, consulting fees and research support from Merck, and shares the patent on the herpes zoster vaccine. He has also received research support from GlaxoSmithKline (GSK). John Litt has received travel support and conference registration fees from CSL to attend Infectious Diseases Society of America and varicella-zoster virus meetings in Toronto and Australia. David Gronow has received honoraria for lectures from Mundipharma, Pfizer and Janssen-Cilag, and is a member of advisory boards for Mundipharma, CSL and Medtronic. Judith Breuer has received funding from GSK, Merck Sharpe & Dohme, Sanofi Pasteur, the Wellcome Trust and the United Kingdom Medical Research Council for work on varicella-zoster vaccines. Dominic Dwyer and Anthony Cunningham participate in clinical trials of antiviral agents and vaccines and sit on advisory boards for pharmaceutical companies that produce drugs and vaccines mentioned in this article.
- 1. Gnann J Jr, Whitley R. Herpes zoster. N Engl J Med 2002; 347: 340-346.
- 2. International Herpes Management Forum. Johnson RW, Whitely R, editors. Combating varicella zoster virus-related diseases. Herpes 2006; 13 Suppl 1: 1-41.
- 3. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352: 2271-2284.
- 4. Gidding HF, MacIntyre CR, Burgess MA, et al. The seroepidemiology and transmission dynamics of varicella in Australia. Epidemiol Infect 2003; 131: 1085-1089.
- 5. MacIntyre CR, Chu CP, Burgess MA. Use of hospitalisation and pharmaceutical prescribing data to compare the revaccination burden of varicella and herpes zoster in Australia. Epidemiol Infect 2003; 131: 675-682.
- 6. Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med 1965; 58: 1-12.
- 7. Ragozzino MW, Melton LF, Kurland LT. Population based study of herpes zoster and its sequelae. Medicine 1982; 61: 310-316.
- 8. Brisson M, Edmunds W, Law B, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect 2001; 127: 305-314.
- 9. Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44 Suppl 1: S1-S26.
- 10. Heininger U, Seward JF. Varicella. Lancet 2006; 368: 1365-1376.
- 11. Dwyer DE, Cunningham AL. Herpes simplex and varicella-zoster virus infections. Med J Aust 2002; 177: 267-273.
- 12. Portenay RK, Duma C, Foley KM. Acute herpetic and postherpetic neuralgia: clinical review and current management. Ann Neurol 1986; 20: 651-664.
- 13. Rawlinson WD, Dwyer DE, Gibbons VL, Cunningham AL. Rapid diagnosis of varicella-zoster virus infection with a monoclonal antibody based direct immunofluorescence technique. J Virol Methods 1989; 23: 13-18.
- 14. Wood M, Kay R, Dworkin RH, et al. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis 1996; 22: 341-347.
- 15. Beutner K, Friedman D, Forszpaniak C, et al. Valaciclovir compared with aciclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother 1995; 39: 1546-1553.
- 16. Tyring S, Barbarash R, Nahlik J, et al. Famciclovir for the treatment of acute herpes zoster: effects on the acute disease and post herpetic neuralgia: a randomised, double-blind, placebo-controlled trial. Ann Intern Med 1995; 123: 89-96.
- 17. Wood MJ, Shukla S, Fiddian AP, Crooks RJ. Treatment of acute herpes zoster: effect of early (< 48 h) versus late (48–72 h) therapy with acyclovir and valaciclovir on prolonged pain. J Infect Dis 1998; 178 Suppl 1: S81-S84.
- 18. Whitely R, Weiss H, Gnann J Jr, et al. Aciclovir with and without prednisone for the treatment of herpes zoster. A randomised, placebo-controlled trial. The National Institute of Allergy and Infectious Disease Collaborative Antiviral Study Group. Ann Intern Med 1996; 125: 376-383.
- 19. Wood M, Johnson R, McKendrick M, et al. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med 1994; 330: 896-900.
- 20. Board of the Australian Herpes Management Forum. Managing herpes zoster: guidelines for clinicians. 2006. http://www.ahmf.com.au/health_professionals/guidelines/managing_herpes_zoster.htm (accessed Mar 2007).
- 21. International Association for the Study of Pain (IASP) Task Force on Taxonomy. Pain terms: a current list of definitions and notes on usage. In: Merskey H, Bogduk N, editors. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. Seattle: IASP Press, 1994: 209-214.
- 22. Neurology Expert Group. Postherpetic neuralgia. In: Therapeutic guidelines: neurology. Version 3. Melbourne: Therapeutic Guidelines Limited, 2007: 163-165.
- 23. National Heath and Medical Research Council. The Australian immunisation handbook. Part 2: Vaccination for special risk groups. 8th ed. Canberra: NHMRC, 2003: 69. http://www9.health.gov.au/immhandbook/ (accessed Aug 2007).
- 24. Kerzner B, Murray AV, Cheng E, et al. Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older. J Am Geriatr Soc 2007; 55: 1499-1507.
- 25. Lolekha S, Tanthiphabha W, Sornchai P, et al. Effect of climatic factors and population density on VZV epidemiology within a tropical country. Am J Trop Med Hyg 2001; 64: 131-136.
- 26. Macaladad N, Marcano T, Guzman M, et al. Safety and immunogenicity of a zoster vaccine in varicella-zoster virus seronegative and low-seropositive healthy adults. Vaccine 2007; 25: 2139-2144.
- 27. US Advisory Committee on Immunization Practices. Provisional Recommendations. Use of shingles (herpes zoster) vaccine. Atlanta, Ga: ACIP, 2006. http://www.cdc.gov/vaccines/recs/provisional/downloads/zoster-11-20-06.pdf (accessed Aug 2007).





Abstract
The burden of illness from herpes zoster (HZ) and postherpetic neuralgia (PHN) in the Australian community is high.
The incidence and severity of HZ and PHN increase with age in association with a progressive decline in cell-mediated immunity to varicella-zoster virus (VZV).
Antiviral medications (valaciclovir, famciclovir, aciclovir) have been shown to be effective in reducing much but not all of the morbidity associated with HZ and PHN, but are consistently underprescribed in Australia.
Zoster-associated pain should be treated early and aggressively, as it is more difficult to treat once established. Clinicians should be proactive in their follow-up of individuals at high risk of developing PHN, and refer patients to a specialist pain clinic earlier, rather than later.
A live, attenuated VZV vaccine (Oka/Merck strain, Zostavax [Merck Sharp & Dohme]) has proven to be efficacious in reducing the incidence of and morbidity associated with HZ and PHN in older adults.
The vaccine’s efficacy has been shown to persist for at least 4 years, but is likely to last a lot longer. Ongoing surveillance will determine the duration of protection and whether a booster dose is required.
Clinicians should consider recommending the vaccine, which can be safely administered at the same time as the inactivated influenza vaccine, to all immunocompetent patients aged 60 years or older.
Clinicians should refer to the Australian immunisation handbook for advice on the use of the live vaccine in immunosuppressed individuals.