During the 2009 southern hemisphere winter, testing for influenza initially overwhelmed reference laboratory resources.1 Most of this initial testing was for non-hospitalised patients, but, as the pandemic (H1N1) 2009 influenza outbreak progressed, the demand for influenza testing (as well as testing for respiratory viruses in general) in non-reference public laboratories for patients admitted to hospital increased dramatically.2
The first locally acquired Australian case of pandemic influenza was confirmed on 22 May 2009.3 By 17 June 2009, there was sustained community transmission and the purpose of testing for influenza changed from public health containment to early detection and treatment of those at risk of severe influenza-related illness, particularly those hospitalised with an influenza-like illness.4 Influenza activity peaked in New South Wales in early July.2
Prospective studies identifying clinical factors that predict influenza infection have mainly been conducted in ambulatory care settings and have shown that — although it is difficult to confirm or exclude influenza on clinical grounds — fever, cough and acute onset are, at least in adults, useful clinical features.5 The hospital and mortality burden of influenza is well recognised,6 but studies aimed at identifying clinical features that predict influenza infection in hospital settings, which could guide rational testing and empiric treatment, are scarce. The epidemic situation in NSW during winter 2009 provided a natural opportunity to study questions related to influenza testing in patients admitted to hospital, including which factors influence clinicians’ decisions to test for influenza and whether detection of influenza could be better predicted.
Two public hospital laboratories within the Sydney South West Pathology Service — at Liverpool Hospital and Royal Prince Alfred Hospital — performed all the influenza testing. Diagnostic testing for influenza A and pandemic influenza subtyping were similar in both laboratories. Following total nucleic acid extraction from nasopharyngeal swabs, influenza A and pandemic influenza were confirmed by polymerase chain reaction using assays which target the matrix protein (influenza A) and nucleocapsid protein gene segment (pandemic influenza) (Influenza 4, Influenza 6 and Respiratory pathogens 12 Easy-Plex assay kits [AusDiagnostics, Sydney, NSW]).7
The Systematized Nomenclature of Medicine – Clinical Terms (SNOMED CT) admission diagnosis code for each patient was categorised to one of 13 diagnosis categories (Box 1).8 These included four main categories (respiratory conditions, fever, cardiac conditions and other conditions). Respiratory conditions were further divided into 10 diagnosis subcategories.
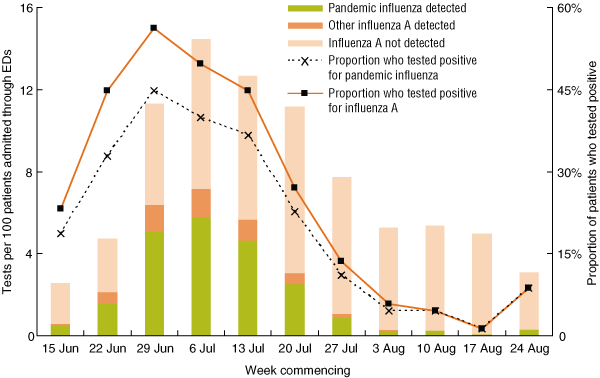
Testing rates varied with time, and the highest testing rate occurred in the period 6 July to 12 July (Week 4), when 254 of 1639 patients (15.5%) were tested (Box 2). The influenza A detection rate peaked in the period 29 June to 5 July (Week 3), when influenza A was detected in 113 of 201 patients (56.2%) who were tested (Box 2).
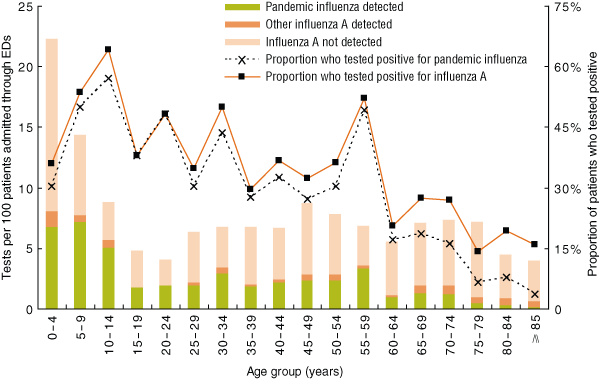
Rates of testing for influenza A and rates of detection of both influenza A and pandemic influenza varied by age group (Box 3). Although the proportion of patients tested was highest in the 0–4-years age group (299 of 1342; 22.3%), the rate of influenza A detection was highest in children aged 10–14 years (18 of 28; 64.3%). Older patients (≥ 60 years) were less likely to be infected with pandemic influenza than younger patients (Box 3).
The proportion of patients who were tested for influenza A varied by diagnosis category (Box 4), with testing rates highest for patients with fever (28.1% tested) or a respiratory condition (27.3% tested). Among these patients, influenza A was most commonly detected in those diagnosed with influenza-like illness (59.1%), viral illness (50.0%) or a fever (48.4%). Within the other respiratory categories, 31.1% of those diagnosed with pneumonia and 30.2% with shortness of breath tested positive, while influenza A was relatively uncommon among patients with bronchiolitis (17.5%) (Box 4).
Multivariate regression analysis (Box 5) showed that diagnosis category had the strongest association with testing for influenza A. For patients with a febrile or a respiratory condition, the odds of being tested were 15 to 17 times higher compared with patients who did not have a febrile, respiratory or cardiac condition at admission. Controlling for age and diagnosis category, the odds of a patient being tested in the period 6 July to 12 July (Week 4) were sevenfold higher than for 15 June to 21 June (Week 1). Week of admission had by far the strongest association with detection of influenza A. The odds of a positive influenza A result were 120 times higher in the period 29 June to 5 July (Week 3) compared with the period 17 August to 23 August (Week 10).
In a case series of patients admitted to hospital with confirmed influenza A during the 2009 southern hemisphere winter, classic features such as cough and fever were commonly reported, but the spectrum of illness was broad.9 Risk factors for severe disease, including pregnancy, airways disease, diabetes and high body mass, have been reported in an intensive care unit case series10 and an epidemiological summary of the first winter wave of the pandemic influenza outbreak.2 Our observational study design, in which two routine datasets were matched for a large sample of patients, enabled us to make broad conclusions about the approach of clinicians to testing for influenza and the features predictive of a positive test result. It only included patients who were admitted to hospital through EDs, hence the results may not be applicable to outpatients and to patients in non-acute-care settings such as general practice. In addition, clinical information, in the form of the SNOMED CT diagnosis codes, was provided at triage. Many of these codes only provide syndromic descriptions of the reason for admission, and the diagnosis codes entered by ED staff at the time of patient admission may not always accurately capture the final diagnosis.11 Nevertheless, the decision to test for influenza is usually made shortly after presentation; therefore, the diagnosis at admission is arguably a more accurate representation of the information available to the clinicians when they decide whether to test for influenza.
So, what factors were most predictive of a positive influenza test result? Week of admission had the strongest association with influenza A detection, outweighing age and diagnosis category. The strong seasonality of influenza is well described,12,13 but the strength of this association is surprising. Although testing intensity was highest in children under 10 years of age, the highest rates of influenza detection were in 10–14-year-old children. This concurs with epidemiological data suggesting a higher incidence of pandemic influenza infection in school-aged children.2,14-16 Similarly, the lower rates of testing and detection in older patients, especially those 80 years and older, suggests a low incidence of disease in older patients, possibly due to pre-existing immunity.2,14,17-21
After controlling for age and week of admission, the association between diagnosis at admission and a positive test result was weak. A diagnosis of fever or a respiratory condition (excluding the category “other respiratory condition”) increased the odds of a positive test result. However, only an influenza-like illness diagnosis stood out in both univariate and logistic regression analyses, with a 59.1% rate of influenza A detection and sevenfold higher odds of a positive influenza test result compared with cardiac conditions. Rates of testing of patients with non-respiratory and non-febrile conditions were low (2.2%–3.1%), but detection rates for these patients were comparable to respiratory diagnosis categories. This result highlights the varying presentations of influenza. In addition, it confirms the insensitivity of any particular influenza case definition, because the broadest definition (anyone with fever or a respiratory condition) identified 81.9% (361/441) of influenza cases in our study. Thus, to prevent hospital transmission, especially in patients at risk of severe influenza-related illness (such as those in haematology and oncology wards), strict infection-control procedures are required for all patients.22,23
So, what should be the rational approach to influenza testing for patients who are admitted to hospital for acute care? First, we suggest that there could be a role for intermittent laboratory testing to complement existing sources of surveillance information.24 Documenting the upswing, peak and tail of the outbreak with active tracking of testing and detection rates could be a cost-effective method to guide clinical management of influenza, particularly the use of anti-influenza therapy.
1 Admission diagnosis categories and examples of Systematized Nomenclature of Medicine – Clinical Terms (SNOMED CT) diagnosis codes for each category
2 Rates of testing for and detection of influenza A in patients admitted through EDs in the SSWAHS, and proportions of patients who tested positive, by influenza A subtype and week of specimen collection, 15 June to 30 August 2009
 |
|
ED = emergency department. SSWAHS = Sydney South West Area Health Service. |
3 Rates of testing for and detection of influenza A in patients admitted through EDs in the SSWAHS, and proportions of patients who tested positive, by influenza A subtype and age group, 15 June to 30 August 2009
 |
|
ED = emergency department. SSWAHS = Sydney South West Area Health Service. |
4 Patients admitted through emergency departments in the Sydney South West Area Health Service who were tested for influenza A and who tested positive for influenza A , by diagnosis at admission, 15 June to 30 August 2009
5 Association between age group, week of admission and diagnosis category and rates of testing for and detection of influenza A in patients admitted through EDs in the Sydney South West Area Health Service, 15 June to 30 August 2009
* Adjusted for clustering by admitting hospital. † Reference category. |
|||||||||||||||
- Andrew Jardine1
- Stephen J Conaty1
- Michelle A Cretikos1,2
- Wei-Yuen Su3
- Iain B Gosbell4,5
- Sebastiaan J van Hal5
- 1 Public Health Unit, Sydney South West Area Health Service, Sydney, NSW.
- 2 School of Public Health, University of Sydney, Sydney, NSW.
- 3 Department of Microbiology, Sydney South West Pathology Service — Royal Prince Alfred Hospital, Sydney, NSW.
- 4 Microbiology and Infectious Diseases Unit, School of Medicine, University of Western Sydney, Sydney, NSW.
- 5 Department of Microbiology and Infectious Diseases, Sydney South West Pathology Service — Liverpool Hospital, Sydney, NSW.
We thank the Public Health Real-time Emergency Department Surveillance System team at NSW Health, and the ED and laboratory staff of SSWAHS.
None identified.
- 1. Grayson ML, Johnson PD. Australia’s influenza containment plan and the swine flu epidemic in Victoria. Med J Aust 2009; 191: 150. <MJA full text>
- 2. New South Wales public health network. Progression and impact of the first winter wave of the 2009 pandemic H1N1 influenza in New South Wales, Australia. Euro Surveill 2009; 14 (42): pii = 19365.
- 3. Australian Government Department of Health and Ageing. Alert level raised to ‘CONTAIN’ [media release]. 22 May 2009. http://www.healthemergency.gov.au/internet/healthemergency/publishing.nsf/Content/news-022 (accessed Sep 2009).
- 4. Australian Government Department of Health and Ageing. New pandemic phase ‘PROTECT’ [media release]. 17 Jun 2009. http://www.healthemergency.gov.au/internet/healthemergency/publishing.nsf/Content/news-170609 (accessed Sep 2009).
- 5. Call SA, Vollenweider MA, Hornung CA, et al. Does this patient have influenza? JAMA 2005; 293: 987-997.
- 6. Simonsen L, Fukuda K, Schonberger LB, Cox NJ. The impact of influenza epidemics on hospitalizations. J Infect Dis 2000; 181: 831-837.
- 7. Szewczuk E, Thapa K, Anninos T, et al. Rapid semi-automated quantitative multiplex tandem PCR (MT-PCR) assays for the differential diagnosis of influenza-like illness. BMC Infect Dis 2010; 10: 113.
- 8. International Health Terminology Standards Development Organisation. SNOMED CT. http://www.ihtsdo.org/snomed-ct (accessed Sep 2009).
- 9. Denholm JT, Gordon CL, Johnson PD, et al. Hospitalised adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. Med J Aust 2010; 192: 84-86. <MJA full text>
- 10. ANZIC Influenza Investigators; Webb SA, Pettilä V, Seppelt I, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 2009; 361: 1925-1934.
- 11. Nachimuthu SK, Lau LM. Practical issues in using SNOMED CT as a reference terminology. Stud Health Technol Inform 2007; 129: 640-644.
- 12. Lipsitch M, Viboud C. Influenza seasonality: lifting the fog. Proc Natl Acad Sci U S A 2009; 106: 3645-3646.
- 13. Lofgren E, Fefferman NH, Naumov YN, et al. Influenza seasonality: underlying causes and modeling theories. J Virol 2007; 81: 5429-5436.
- 14. Kelly HA, Grant KA, Williams S, et al. Epidemiological characteristics of pandemic influenza H1N1 2009 and seasonal influenza infection. Med J Aust 2009; 191: 146-149. <MJA full text>
- 15. Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 2009; 302: 1896-1902.
- 16. Torres J, O’Ryan M, Herve B, et al. Impact of the novel influenza A (H1N1) during the 2009 autumn–winter season in a large hospital setting in Santiago, Chile. Clin Infect Dis 2010; 50: 860-868.
- 17. Bishop JF, Murnane MP, Owen R. Australia’s winter with the 2009 pandemic influenza A (H1N1) virus. N Engl J Med 2009; 361: 2591-2594.
- 18. Ikonen N, Strengell M, Kinnunen L, et al. High frequency of cross-reacting antibodies against 2009 pandemic influenza A(H1N1) virus among the elderly in Finland. Euro Surveill 2010; 15 (5): pii: 19478.
- 19. Miller E, Hoschler K, Hardelid P, et al. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet 2010; 375: 1100-1108.
- 20. Pan C, Cheung B, Tan S, et al. Genomic signature and mutation trend analysis of pandemic (H1N1) 2009 influenza A virus. PLoS One 2010; 5: e9549.
- 21. Ross T, Zimmer S, Burke D, et al. Seroprevalence following the second wave of pandemic 2009 H1N1 influenza. PLoS Curr Influenza 2010; Feb 24: RRN1148.
- 22. Centers for Disease Control and Prevention (CDC). Interim recommendations for clinical use of influenza diagnostic tests during the 2009–10 influenza season. Atlanta: CDC, 2009.
- 23. van Hal S, Foo H, Blyth C, et al. Influenza outbreak during Sydney World Youth Day 2008: the utility of laboratory testing and case definitions on mass gathering outbreak containment. PLoS One 2009; 4: e6620.
- 24. Muscatello DJ, Churches T, Kaldor J, et al. An automated, broad-based, near real-time public health surveillance system using presentations to hospital Emergency Departments in New South Wales, Australia. BMC Public Health 2005; 5: 141.





Abstract
Aim:
Design, setting and participants: Retrospective observational study of patients who were tested for influenza A after being admitted to hospital through emergency departments of the Sydney South West Area Health Service from 15 June to 30 August 2009.
Main outcome measures: The association of factors such as age, diagnosis at admission, hospital and week of admission with rates of testing and detection of influenza A.
Results: 17 681 patients were admitted through nine emergency departments; 1344 (7.6%) were tested for influenza A, of whom 356 (26.5%) tested positive for pandemic influenza. Testing rates were highest in 0–4-year-old children, in the peak period of the outbreak, and in patients presenting with a febrile or respiratory illness. Positive influenza test results were common across a range of diagnoses, but occurred most frequently in children aged 10–14 years (64.3%) and in patients with a diagnosis at admission of influenza-like illness (59.1%). Using multivariate logistic regression, patients with a diagnosis at admission of fever or a respiratory illness at admission were most likely to be tested (odds ratios [ORs], 15 [95% CI, 11–21] and 17 [95% CI, 15–19], respectively). These diagnoses were stronger predictors of influenza testing than the peak testing week (Week 4; OR, 7.0 [95% CI, 3.8–13]) or any age group. However, diagnosis at admission and age were significant but weak predictors of a positive test result, and the strongest predictor of a positive test result was the peak epidemic week (Week 3; OR, 120 [95% CI, 27–490]).
Conclusion: The strongest predictor of a clinician’s decision to test for influenza was the diagnosis at admission, but the strongest predictor of a positive test was the week of admission. A rational approach to influenza testing for patients who are admitted to hospital for acute care could include active tracking of influenza testing and detection rates, testing patients with a strong indication for antiviral treatment, and admitting only those who test negative to “clean” wards during the peak of an outbreak.