In his recent clinical update, Senanayake discusses the epidemiology of pandemic influenza H1N1 2009, colloquially known as “swine flu”.1 Senanayake describes features of infection caused by the pandemic virus and refers to our recent study comparing the median ages of patients with pandemic and seasonal influenza infection.2 However, Senanayake’s statement that a predilection for younger age groups may be a feature of all influenza A viruses is a misinterpretation of our data. What we showed was a tendency for a younger median age of infection with seasonal influenza A(H1N1) and influenza B virus compared with seasonal influenza A(H3N2). This is illustrated using data from polymerase chain reaction-confirmed influenza infections detected in sentinel surveillance of patients with influenza-like illness (ILI) in Victoria and Western Australia in 2007 and 2008 (Box 1). The median age of patients with seasonal influenza A(H1N1) infection was 8–13 years lower than the median age of those with influenza A(H3N2), while the median age of patients with influenza B infection was similar to that for influenza A(H1N1).
We therefore hypothesised that a younger median age was a feature of influenza A(H1N1) infection, whether seasonal or pandemic, but not of all influenza A infections.2 The younger median age of patients with influenza A(H1N1) infection has also been shown in a recent study that is yet to be published.3 Using publicly available information from multiple geographic regions, the authors showed that symptomatic infection due to seasonal influenza A(H3N2) is distributed across all age groups, whereas seasonal influenza A(H1N1) causes symptomatic disease mainly in a younger population.
We also examined the median age of patients with influenza infection who were tested as part of routine diagnosis in Victoria and WA in 2007 and 2008. While the median ages were different in the two states, reflecting different referral patterns, the median age of patients with influenza A(H3N2) was consistently 8–9 years higher than those with influenza A(H1N1) infections.2
Our hypothesis of similar median ages of patients with pandemic influenza H1N1 2009 and seasonal influenza H1N1 infection seemed reasonable during the early stage of the pandemic in the United States and Europe, when the median age of infection was reported as being in the range of 20–25 years.4,5 However, as the pandemic has unfolded, some countries are reporting a lower median age of infection. Although the median age of patients in the US was reported as 20 years for the first 642 cases,4 a subsequent review of more than 10 000 cases recorded a median age of 13 years (Lyn Finelli, US Centers for Disease Control and Prevention [CDC], personal communication). However, rather than reflecting a real change in age distribution, the lower reported median age may reflect bias caused by increased testing in school children — testing that might not have been performed in previous influenza seasons.
The interim data on the median age of patients with pandemic influenza infection in Victoria and WA support our previous observation about the similarity of the median age of patients with pandemic H1N1 influenza infection in Europe and the US in 2009 and those with seasonal influenza A(H1N1) in Australia in 2007 and 2008 (Box 1). Analysis of all notified cases of pandemic influenza H1N1 2009 in Victoria from the database maintained by the Victorian Department of Human Services, which includes cases from all outbreak investigations and contact tracing, shows that the median age of the first 1000 patients notified in Victoria was 15 years. This lower median age may reflect the same testing bias seen in the larger dataset from the US (ie, differentially increased testing of school-aged children relative to their attack rate).
For instance, there are few reliable data on the community attack rate for seasonal influenza, and even fewer data on variations in attack rate by influenza type and subtype. Although US studies based on serological confirmation of influenza in Tecumseh and Seattle in the 1970s estimated attack rates of up to 20%,6 commonly quoted estimates of seasonal influenza attack rates vary between 5% and 10%.7 However, data from an Australian survey of general practice suggest the rate may be as low as 1%,8 especially in years of relatively low activity. This lower rate is consistent with surveillance data over more than a decade from the Netherlands, England and Wales.9 Attack rates and outcomes are different for different subtypes. Influenza A(H3N2) is thought to be associated with worse outcomes10 and affects an older age group,2 whereas the attack rate for seasonal influenza A(H1N1) may be higher because of spread among children.6 Influenza B affects a younger age group than influenza A(H3N2) and is generally associated with less severe outcomes.
We also know little about deaths due to laboratory-confirmed influenza, estimates for which are derived from modelling rather than testing. In Australia, it is thought that an average of about 3000 excess deaths each year may be attributed to influenza infection in people aged at least 50 years — the age group in which most deaths occur.11 Indeed, at least 85% of these deaths occur in people aged 65 years or older, many of whom have underlying conditions that render them more susceptible to adverse outcomes. Using modelled estimates of the number of deaths in conjunction with a range of plausible attack rates in Australia, the case-fatality ratio (CFR) for seasonal influenza might vary between 0.14% (with an attack rate of 10%) and 1.4% (with an attack rate of 1%). The latter CFR seems implausible and reflects general uncertainty about both attack rates and attributable deaths. Early estimates for the CFR for pandemic influenza in the US were more like the lower end of this range,4 but the number of US cases used to estimate the CFR is likely to be substantially underestimated, suggesting the real CFR is lower (Lyn Finelli, US CDC, personal communication). As testing patterns change, with an increasing emphasis on detecting more severe cases and lower capture of all cases, the apparent CFR is likely to rise in all countries. A reliable estimate of the real CFR, for both seasonal and pandemic influenza, remains elusive.
The age of people who have become seriously ill or died from laboratory-confirmed pandemic influenza during the early phase of the pandemic is younger than the age of those modelled to die from seasonal influenza. This age difference is highlighted in a study showing that 87% of severe pneumonia deaths in Mexico between 24 March and 29 April 2009, when pandemic influenza H1N1 virus was circulating, occurred in those aged 5–59 years, compared with 17% of deaths in this age group in the influenza seasons of 2006–2008.12 All deaths were associated with influenza virus circulation but were not shown to be caused by laboratory-confirmed influenza.
Although not always the case in Mexico in March–April 2009, adult patients hospitalised with severe pneumonia in Australia are currently being tested for pandemic influenza. However, comparison between hospitalisations for seasonal and pandemic influenza will be difficult because the estimate of 18 400 hospital admissions attributable to influenza annually in Australia is also derived from modelling rather than laboratory testing.11 With the current increase in laboratory testing, we have a valuable opportunity to gain a greater insight into the impact of seasonal influenza, given that surveillance data from Victoria13 and WA14 indicate co-circulation of the novel and seasonal influenza viruses in May and the first weeks of June.
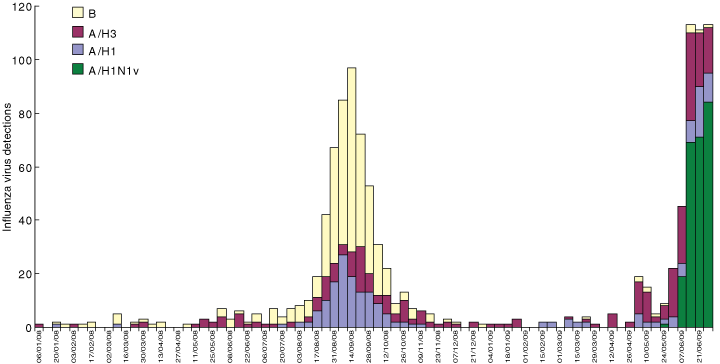
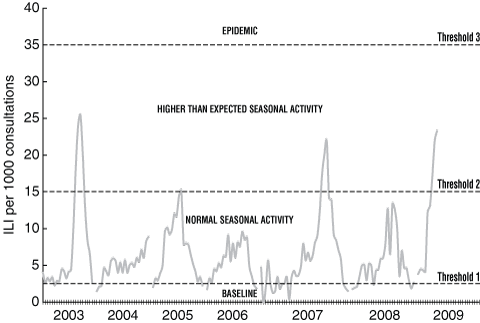
However, we believe that WA and Victoria are at different stages of the pandemic, with earlier community transmission and wider spread of the novel virus in Victoria than in WA. Influenza virus detections from samples referred to the PathWest Medical Laboratory at the Queen Elizabeth II Medical Centre in WA during 2008 and up to the end of June 2009 show high numbers of pandemic influenza H1N1 infections by mid June, but with other seasonal influenza viruses still circulating (Box 2). Sentinel surveillance data from Victoria for 2003–2009 show a rapid early increase in the ILI rate in 2009 (Box 3). Moreover, the proportion of patients with ILI who tested positive for influenza increased from 6% in the first week of surveillance to 63% in the ninth week and, where subtyping data were available, the proportion of influenza detections identified as pandemic influenza H1N1 2009 increased from none to 99% during the same period (Box 4).
The increased testing associated with the response to the pandemic highlights how little diagnostic testing is done for seasonal influenza, a fact that has ramifications for our understanding of public policy relating to influenza control. As an example of this, the lack of data on laboratory-confirmed seasonal influenza has led to arguments about the relative effectiveness of influenza vaccine in the prevention of all-cause mortality.15 We have argued, as have Simonsen and colleagues,15 that the estimated 50% vaccine effectiveness against all-cause mortality provided by influenza vaccine is not credible, and that the real vaccine effectiveness against this non-specific outcome should be in the order of 2.5%–5%.16 This argument could potentially be resolved if there were increased laboratory testing of adult patients hospitalised with a wide range of cardiovascular and respiratory diseases, and if these patients were followed up to assess outcomes, including mortality. With the increased laboratory testing that has resulted from Australia’s response to the influenza H1N1 2009 pandemic, we have a potential opportunity to better understand the impact of seasonal influenza by analysing community infection, hospitalisations and deaths caused by laboratory-confirmed infections.
1 Median age of patients with seasonal influenza infection, by type and subtype, identified through sentinel surveillance in Victoria and Western Australia, 2007–2008
2 Influenza virus detections, by type and subtype, in samples referred to the PathWest Medical Laboratory at the Queen Elizabeth II Medical Centre, Western Australia, 2008–2009
3 Rate of influenza-like illness (ILI) per 1000 consultations in patients from general practice sentinel surveillance in Victoria, 2003–2009
- Heath A Kelly1
- Kristina A Grant1
- Simon Williams2
- James Fielding3
- David Smith2
- 1 Epidemiology Unit, Victorian Infectious Diseases Reference Laboratory, Melbourne, VIC.
- 2 PathWest Laboratory Medicine, Perth, WA.
- 3 Communicable Disease Prevention and Control Unit, Victorian Department of Human Services, Melbourne, VIC.
None identified.
- 1. Senanayake SN. Swine flu update: bringing home the bacon. Med J Aust 2009; 191: 138-140 [Published online ahead of print, 17 Jun 2009] <eMJA full text>.
- 2. Kelly H, Grant K, Williams S, Smith D. H1N1 swine origin influenza infection in the United States and Europe in 2009 may be similar to H1N1 seasonal influenza infection in two Australian states in 2007 and 2008. Influenza Other Respi Viruses 2009; 3: 183-188. Epub 2009 Jun 8.
- 3. Khiabanian H, Farrell GM, St George K, Rabadan R. Differences in patient age distribution between influenza A subtypes. 30 May 2009. Columbia University Libraries Academic Commons. http://app.cul.columbia.edu:8080/ac/handle/10022/AC:P:29810 (accessed Jul 2009).
- 4. Dawood FS, Jain S, Finelli L, et al; Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360: 2605-2615. Epub 2009 May 7.
- 5. Surveillance Group for New Influenza A(H1N1) Virus Investigation and Control in Spain. New influenza A(H1N1) virus infections in Spain, April–May 2009. Euro Surveill 2009; 14 (19): pii=19209.
- 6. Longini IM Jr, Koopman JS, Monto AS, Fox JP. Estimating household and community transmission parameters for influenza. Am J Epidemiol 1982; 115: 736-751.
- 7. Newall AT, Kelly H, Harsley S, Scuffham PA. Cost-effectiveness of influenza vaccination in older adults: a critical review of economic evaluations targeting the 50–64 year age group. Pharmacoeconomics 2009. In press.
- 8. Newall AT, Scuffham PA. Influenza-related disease: the cost to the Australian healthcare system. Vaccine 2008; 26: 6818-6823.
- 9. Fleming DM, Zambon M, Bartelds AI, de Jong JC. The duration and magnitude of influenza epidemics: a study of surveillance data from sentinel general practices in England, Wales and the Netherlands. Eur J Epidemiol 1999; 15: 467-473.
- 10. Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine 1999; 17 Suppl 1: S3-S10.
- 11. Newall AT, Wood JG, Macintyre CR. Influenza-related hospitalisation and death in Australians aged 50 years and older. Vaccine 2008; 26: 2135-2141.
- 12. Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009 Jun 29. [Epub ahead of print.]
- 13. Victorian Infectious Diseases Reference Laboratory. Sentinel influenza surveillance. http://www.vidrl.org.au/surveillance/flu%20reports/flu_idx.html (accessed Jul 2009).
- 14. Communicable Disease Control Directorate, Government of Western Australia Department of Health. Virus Watch. http://www.public.health.wa.gov.au/3/487/3/virus_watch_homepage.pm (accessed Jul 2009).
- 15. Simonsen L, Taylor RJ, Viboud C, et al. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 2007; 7: 658-666.
- 16. Kelly H, Newall AT. Mortality benefits of influenza vaccination in elderly people. Lancet Infect Dis 2008; 8: 462-463; author reply 463-465.





Abstract
The median age of patients with seasonal influenza A(H1N1) infection identified through sentinel surveillance in Western Australia and Victoria in 2007–2008 was 18 and 22 years, respectively. For pandemic influenza H1N1 2009 infection, the median age of the first 244 patients identified in WA was 22 years, and median age of the first 135 patients identified through sentinel surveillance in Victoria was 21 years.
Other comparisons of the epidemiological features of pandemic and seasonal influenza are difficult because much less laboratory testing is done for seasonal than for pandemic influenza.
While early surveillance data indicated co-circulation of both pandemic and seasonal strains in WA and Victoria, more recent data from both states indicate an increasing predominance of pandemic influenza.
If the evolving pandemic allows, we should take advantage of the increased testing being conducted for pandemic influenza to learn more about the real impact of laboratory-confirmed seasonal influenza.