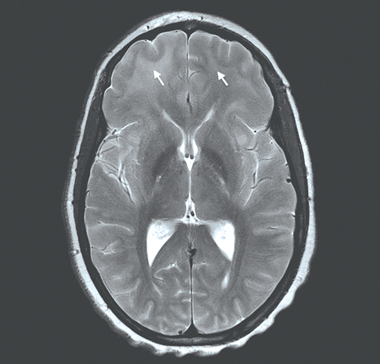
Persistent status epilepticus necessitated treatment with loading doses of phenytoin, sedation and intubation. Magnetic resonance imaging (MRI) of the brain showed extensive areas of uniform high signal intensity within the supratentorial white matter and mild meningeal enhancement (Box 1). Meningoencephalitis was diagnosed, and the patient was treated with ceftriaxone and aciclovir. Based on history, presentation and cerebrospinal fluid findings, she was also given isoniazid, rifampicin, ethambutol, pyrazinamide, pyridoxine and dexamethasone for suspected tuberculous meningoencephalitis. Multiple investigations for bacterial, viral and fungal pathogens, including serological tests for HIV, and polymerase chain reaction (PCR) tests and culture for Mycobacterium tuberculosis, gave negative results (Box 2).
The patient was extubated on Day 4, and her condition improved, with no further seizures, although periods of fluctuating drowsiness and confusion were noted. Electroencephalography showed generalised slowing consistent with diffuse cerebral dysfunction. To clarify the diagnosis, lumbar puncture was repeated; CSF showed polymorphonuclear leukocytes, 0 × 106/L; mononuclear cells, 141 × 106/L; protein, 0.52 g/L; and glucose, 2.0 mmol/L; but was again Gram stain-negative. Investigations for autoimmune and malignant disease, as well as mitochondrial disorders and metabolic leukodystrophies gave negative results (Box 2).
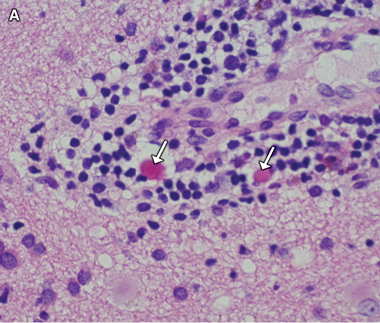
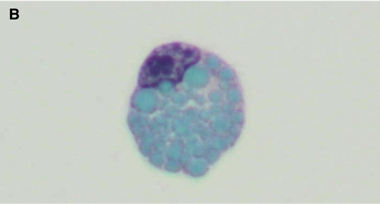
A diagnosis of West African trypanosomiasis was considered, but peripheral blood and lymph node tissue were negative for trypanosomes; lumbar puncture was not performed because of concern about increased intracranial pressure. Because of a rapid deterioration in the patient’s condition and the continuing uncertainty over the diagnosis, a stereotactic brain biopsy was performed. Histological examination of the biopsy specimen showed meningoencephalitis with Mott (morula) cells (Box 3A), suggesting human African trypanosomiasis (HAT). Review of the previous CSF cytology specimens also revealed Mott cells (Box 3B), which had not been recognised initially. Indirect haemagglutination tests of serum for HAT-specific antibodies gave positive results, with a titre of 1:4096.
2 Summary of investigations undertaken with negative results*
Bacteria. Brucella abortus (serum agglutination titre), meningococcus (PCR), mycobacterium (PCR, culture), mycoplasma (PCR), syphilis (serology), streptococcal antigen, rapid screen for bacterial antigens, and blood, urine, cerebrospinal fluid, sputum and stool cultures. Parasites. Blood parasite screen (including malaria), cryptococcal antigen, schistosomiasis (IgG) and toxoplasma (IgM, IgG). Metabolic screen: Cerebrospinal fluid pyruvate, lysosomal enzymes and very long chain fatty acids. |
HAT is caused by the flagellated protozoan Trypanosoma brucei. Two subspecies cause human disease: T. brucei rhodesiense, which is found only in east Africa and causes an acute, fulminant illness lasting weeks to months (East African HAT); and T. brucei gambiense, which is found in western, central and parts of east Africa, and causes an indolent, chronic infection that may last many months to years (West African HAT).1 The fulminant course of T. b. rhodesiense illness means it is the form more frequently diagnosed outside Africa, in returned travellers.
Our patient probably became infected in Uganda, where both subspecies of trypanosome are known to circulate.2 In retrospect, the initial improvement in her condition was most likely a response to corticosteroid treatment, and the subsequent regression coincided with a reduction in the corticosteroid dose. Cervical lymphadenopathy in the posterior triangle was noted at initial presentation but was recognised only during her second admission as a classic feature of T. b. gambiense disease (the Winterbottom sign3).
Detection of trypanosomes in the CSF, blood or lymph tissue remains the diagnostic “gold standard”. However, methods of parasite detection vary in sensitivity, and the cyclical nature of parasitaemia means parasites are seldom detected in blood, especially in T. b. gambiense disease,4 although detected more often in CSF.5 We were not able to demonstrate trypanosomes in whole blood, lymph node tissue or CSF. In the absence of demonstrable parasites, and where there is clinical suspicion, serological testing is crucial in diagnosis of T. b. gambiense disease. The sensitivity and specificity of currently available serological tests vary from 71% to 100% and 95% to 99%, respectively. In our patient, the high antibody titre supported a diagnosis of active trypanosomal infestation, although titres are known to remain high several years after treatment.6
MRI findings in trypanosomiasis are non-specific, ranging from meningeal enhancement in earlier CNS disease to bilateral confluent hyperintense T2 signals in the subcortical white matter in late disease, as seen in our patient.7 Although not diagnostic, MRI is a useful adjunct in supporting the diagnosis as relatively few conditions cause this distinct MRI appearance, including acute disseminated encephalomyelitis, cerebral gliomatosis, the leukodystrophies and lymphoma, all of which were considered in our patient.
Non-specific CNS findings are meningoencephalitis, comprising leptomeningitis and encephalitis with diffuse perivascular white matter infiltration, microglial nodules and reactive astrocytosis.8 The presence of Mott cells in the CNS and CSF is characteristic of HAT, but these cells are easily overlooked.9 They are morula forms of plasma cells containing prominent eosinophilic cytoplasmic inclusions (Russell bodies) that stain positively with periodic acid-Schiff stain and are composed of immunoglobulin M. Although Mott cells can be found in myeloproliferative disorders, they are considered virtually pathognomonic of HAT in patients with appropriate clinical findings and exposure history.10
Early stage T. b. gambiense disease is treated with pentamidine. Eflornithine, an ornithine decarboxylase inhibitor that interferes with cell division, is the recommended first-line treatment for CNS disease.11 Side effects include seizures, gastrointestinal upset and reversible myelosuppression. As it requires intravenous infusions four times daily over 2 weeks, it is not ideal in resource-constrained settings; recent studies suggest a combination of eflornithine and oral nifurtimox to be as effective but superior in ease of administration and duration of treatment (requiring eflornithine to be given only twice daily for 7 days).12 From May 2009, the WHO has included combination therapy in its essential list of medicines for treatment of West African trypanosomiasis, but was unable to provide nifurtimox for our patient, as its use was approved at that time only for Chagas disease.
To our knowledge, our patient represents the fourth case of HAT identified in Australia. A case of meningoencephalitis thought to be due to T. b. gambiense was diagnosed in Perth in 2008 in an immigrant from Sudan and is also reported in this issue of the Journal13 (page 417). A case due to T. b. rhodesiense was diagnosed at our institution in 1998 in a returned traveller with acute illness after visiting a Tanzanian game park.14 The first case in Australia is said to have occurred after World War II.15
Our patient illustrates the need for clinicians to be aware of HAT as a potential cause of meningoencephalitis, particularly in African immigrants, for months or years after they move to Australia.16 Had this possibility been recognised at presentation and specifically investigated, prompt treatment could have been given, and brain biopsy avoided.
Lessons from practice
Human African trypanosomiasis (HAT) or sleeping sickness is rarely encountered in Australia; physicians should be aware of its possibility in individuals who present with meningoencephalitis and have lived in or visited endemic areas.
Definitive diagnosis relies on parasite detection; in its absence, serological testing is the next most appropriate investigation, particularly for disease cased by Trypanosoma brucei gambiense.
Corticosteroid therapy can mask symptoms of disease and falsely suggest an improvement, confounding the diagnosis.
We recommend that individuals from endemic areas with clinically suspected HAT be screened serologically. This would prevent a potentially fatal delay in diagnosis and permit further monitoring and investigation to confirm and treat disease earlier.
- 1. World Health Organization. African trypanosomiasis fact sheet. Geneva: WHO, 2006.
- 2. Picozzi K, Fevre EM, Odiit M, et al. Sleeping sickness in Uganda: a thin line between two fatal diseases. BMJ 2005; 331: 1238-1241.
- 3. Kirchhoff LV. Trypanosomiasis. In: Kaspar DL, Braunwald E, Fauci AS, et al, editors. Harrison’s principles of internal medicine. Sydney: McGraw-Hill, 2005: 1238-1242.
- 4. Kennedy PG. Human African trypanosomiasis of the CNS: current issues and challenges. J Clin Invest 2004; 113: 496-504.
- 5. Miezan TW, Meda HA, Doua F, et al. Assessment of central nervous system involvement in gambiense trypanosomiasis: value of the cerebro-spinal white cell count. Trop Med Int Health 1998; 3: 571-575.
- 6. Chappuis F, Loutan L, Simarro P, et al. Options for field diagnosis of human African trypanosomiasis. Clin Microbiol Rev 2005; 18: 133-146.
- 7. Kager PA, Schipper HG, Stam J, Majoie CBLM. Case report: magnetic resonance imaging findings in human African trypanosomiasis: a four-year follow-up study in a patient and review of the literature. Am J Trop Med Hyg 2009; 80: 947-952.
- 8. Kennedy PGE. Human African trypanosomiasis: In and out of Africa. Neurology 2006; 66: 962-963.
- 9. Sahlas DJ, MacLean JD, Janevski J, Detsky AS. Clinical problem-solving. Out of Africa. N Engl J Med 2002; 347: 749-753.
- 10. Lejon V, Buscher P. Cerebrospinal fluid in human African trypanosomiasis: a key to diagnosis, therapeutic decision and post-treatment follow-up. Trop Med Int Health 2005; 10: 395-403.
- 11. World Health Organization: Human African trypanosomiasis. http://www.who.int/trypanosomiasis_african/diagnosis/en/index.html (accessed Jan 2010).
- 12. Priotto G, Kasparian S, Mutombo W, et al. Nifurtimox–eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised phase III, non-inferiority trial. Lancet 2009; 374: 7-9.
- 13. Cherian P, Junckerstorff R, Rosen D, et al. Late-stage human African trypanosomiasis in a Sudanese refugee. Med J Aust 2010; 192: 417-419. <MJA full text>
- 14. Maddocks S, O’Brien R. African trypanosomiasis in Australia. N Engl J Med 2000; 342: 1254.
- 15. Darby JD, Huber MGP, Sieling WL, Spelman DW. African trypanosomiasis in two short-term Australian travelers to Malawi. J Travel Med 2008; 15: 375-377.
- 16. Beaman MH, Wesselingh SL. Acute community-acquired meningitis and encephalitis. Med J Aust 2002; 176: 389-396. <MJA full text>





We thank Dr Pere P Simarro (Innovative and Intensified Disease Management Program, Control of Neglected Tropical Diseases Unit of the World Health Organization, Geneva, Switzerland) for providing eflornithine. Both eflornithine and nifurtimox can now be obtained from the WHO. We also thank the patient for permission to report her story.
None identified.