Acute meningitis and encephalitis are medical emergencies that require prompt assessment and treatment.1 We discuss these diseases in the community setting, in the absence of immunocompromised states such as AIDS. Both diseases usually manifest with acute severe headache (often described as the worst ever) and fever. Early distinction between the two conditions is critical, as their management is quite different and delay can have devastating effects.
Acute meningitis causes meningeal inflammation developing over hours to days. Symptoms include headache, photophobia, neck stiffness and, much later, confusion and coma. Disease caused by meningococcus (Neisseria meningitidis) may have a fulminant course over hours and may also occur without meningeal involvement. The presence of confusion, stupor or mental state changes relatively early in the course of the illness suggests encephalitis, which is also less likely to induce neck stiffness and photophobia.
The epidemiology of meningitis varies significantly over time and between locations; up-to-date local Australian information and current management guidelines can be found at the website <http://www.health.gov.au/internet/wcms/Publishing.nsf/Content/cda-pubs-cdi-cdiintro.htm>.2
Symptoms of acute meningitis may be subacute and non-specific. Signs of meningeal irritation are important clues; classical descriptions highlighted the importance of performing several different tests, including the Kernig and Brudzinski signs; the sensitivity of the former is increased with the patient seated.5
This has symptoms similar to those of viral meningitis, although often more severe. Onset is often more sudden than in viral meningitis, with rapidly declining level of consciousness or cranial nerve palsies. In meningococcal meningitis, rash on the extremities may initially be macular but quickly evolves into petechiae and then purpura (Box 1). In young children and the elderly, bacterial meningitis may lack the typical signs of meningism and may present insidiously with lethargy, altered behaviour, confusion or nausea and vomiting.
Diagnostic steps in acute meningitis are outlined in Box 2. This is a simplified diagnostic algorithm; in most cases, consultation with local experts and close liaison with laboratories (to select the most useful tests) is recommended.
Critical to the diagnosis is examination of the cerebrospinal fluid (CSF). Lumbar puncture is safe in the absence of signs of raised intracranial pressure. The CSF profile may help differentiate meningitis from encephalitis and viral from bacterial meningitis (Box 3). However, the CSF profile may vary early in viral infections (with initial neutrophil predominance) and after antibiotic treatment of bacterial meningitis, which may render the Gram stain negative and protein level normal. However, these factors tend not to significantly affect the opening pressure, cell count or CSF : serum glucose ratio. The CSF profile also appears normal at presentation in about 3% of cases of bacterial meningitis.
Lumbar puncture should be performed initially, unless the patient's condition is unstable. In that case, blood should be collected for culture (if not already taken), and empirical antibiotic therapy administered immediately. The need for CT before lumbar puncture is controversial; we suggest that, unless the patient has depressed consciousness, papilloedema or focal neurological signs, lumbar puncture can be performed safely without a prior CT scan (especially if less than 5 mL CSF is collected via a 22-gauge needle).
Empirical antibiotic therapy for acute bacterial meningitis is outlined in Box 4. The incidence of penicillin resistance among clinical isolates of S. pneumoniae in Australia has risen from 1% in 1989 to 25% in 1997,8 with recent studies showing resistance in CSF isolates ranging from 1.4% in Victoria9 to 20.5% in Sydney (Dr P McIntyre, Deputy Director, National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases, The New Children's Hospital, and University of Sydney, Sydney, NSW, personal communication, 2001). In view of this, patients without the typical meningococcal rash should be treated empirically with ceftriaxone plus vancomycin or rifampicin10 (case report, Box 5). Vancomycin failures have been reported, and penetration into CSF is a concern if concurrent steroids have been given. Neither regimen has been subject to controlled clinical trial and recommendations are mainly based on in-vitro11 and animal12 data. Meropenem is available for hospital use and has been used successfully as an alternative, but resistance to this agent is evolving.
Duration of antibiotic therapy for bacterial meningitis is not evidence-based. Ten to 14 days of antibiotics for non-meningococcal meningitis seems reasonable, and seven to 10 days for meningococcal meningitis is appropriate for uncomplicated cases. Shorter courses have been successful in some settings.
Specific antivirals are currently not licensed for treatment of enteroviral meningitis in Australia. However, pleconaril (an antiviral with activity against rhinoviruses and enteroviruses) is available on compassionate use access and should be considered in the management of viral meningitis in patients with immunodeficiency.
An aim of meningitis treatment should be to control the inflammatory response. A recent meta-analysis of dexamethasone use in meningitis showed benefit in management of childhood H. influenzae meningitis.13 Some benefit was demonstrated with its use in the management of pneumococcal meningitis in children. No studies have proven its value in uncomplicated bacterial meningitis in adults or in viral meningitis. Some practitioners (in the absence of data) use corticosteroids in patients with evidence of raised intracranial pressure, focal or lateralising signs or evidence of cerebral oedema on brain imaging.
Chemoprophylaxis for H. influenzae and N. meningitidis aims to prevent secondary disease in close contacts of infected people by eradicating nasopharyngeal colonisation. There is no evidence that chemoprophylaxis for S. pneumoniae is useful. Recommended chemoprophylaxis is shown in Box 6. In general, ceftriaxone is preferred in pregnant women, and ciprofloxacin in men and non-pregnant women.
Encephalitis implies inflammation of the brain substance, parenchyma, which may coexist with inflammation of the meninges (meningoencephalitis) or spinal cord (encephalomyelitis). Encephalitis may be mild and self-limited, or may produce devastating illness. Consultation with an infectious diseases physician or neurologist is recommended in its management. A comprehensive review of this condition was published recently.14
Herpes simplex virus (HSV) is the most common cause of non-seasonal encephalitis in Australia. Without treatment, HSV encephalitis is fatal in up to 80% of cases, and leaves up to 50% of survivors with long-term sequelae.15 In the absence of particular risk factors, other common causes are enteroviruses (including enterovirus type 71, which has recently caused epidemics of meningoencephalitis16), influenza virus and Mycoplasma pneumoniae. However, the likely pathogens in encephalitis are dramatically influenced by geographic location, history of travel and animal exposures (Box 7) and vaccination.
Murray Valley encephalitis (MVE) virus causes seasonal epidemics of encephalitis at times of high regional rainfall.17 This arthropod-borne virus is the most common flavivirus to cause encephalitis in Australia. The epicentre of endemic disease is in the east Kimberley region. Over the past five years, the distribution of Japanese B encephalitis (JE) virus has expanded into Australia via the Torres Strait Islands.19 It causes disease clinically similar to MVE. In addition, two novel encephalitis viruses were recently identified in Australia —Hendra virus20 and Australian bat lyssavirus.21 They should be considered if there is a history of animal exposure (horse or bat, respectively — Box 7), or no other pathogen can be implicated.
Mycobacterium tuberculosis, the yeast C. neoformans and Treponema pallidum (syphilis) may also affect the brain parenchyma, but usually produce chronic or subacute meningitis in such circumstances.
Encephalitis (especially if caused by HSV) may present with progressive headache, fever and alterations in cognitive state (confusion, behavioural change or dysphasia) or consciousness. Focal neurological signs (paresis) or seizures (focal or generalised) may also occur (case report, Box 8). Upper motor signs (hyperreflexia and extensor–plantar responses) are often present, but flaccid paralysis and bladder symptoms may occur if the spinal cord is involved. Associated movement disorders or the syndrome of inappropriate antidiuretic hormone secretion may be seen. Specific clinical clues as to cause are shown in Box 9.
In northern Australia, it may be desirable to distinguish MVE from Japanese encephalitis clinically. Both conditions frequently affect the brainstem and basal ganglia, but MVE often involves the spinal cord, while Japanese encephalitis may produce striking meningeal signs, with or without thalamic involvement. Both have high mortality (25%–33%) and rates of chronic sequelae in survivors (~ 50%).14,17
The major differential diagnosis is meningitis or parameningeal infection, especially if meningitis is caused by atypical pathogens such as Listeria monocytogenes, M. tuberculosis, C. neoformans and, during the wet season in the Northern Territory, Burkholderia pseudomallei.
Other causes of encephalopathy should be considered, including:
metabolic disturbances (hypoglycaemia, hyponatraemia and hypocalcaemia);
thiamine deficiency (Wernicke's encephalopathy);
drugs (including neuroleptics [neuroleptic malignant syndrome], trimethoprim–sulfamethoxazole, isoniazid, non-steroidal anti-inflammatory drugs, intoxications); and
inflammatory disorders, such as Behçet's disease, Reye's and Guillain–Barré syndromes, sarcoidosis, cerebral vasculitis (with or without a cerebral vascular accident), systemic lupus erythematosus and Wegener's granulomatosis.
Status epilepticus and malignancy (paraneoplastic syndrome) may also sometimes be confused with encephalitis.
A combination of magnetic resonance imaging (MRI) and CSF examination narrows the broad differential diagnosis. Brain biopsy is now rarely performed, but still has a place in cases which do not respond to aciclovir. As the diagnosis may be unclear, even after extensive investigation, it is worth storing extra CSF and serum for later analysis (including retrospective testing if new agents of encephalitis are discovered, or new diagnostic tests for existing pathogens become available). A staged approach may save resources, ruling out more important causes initially (HSV, enteroviruses, M. tuberculosis and C. neoformans) and rarer causes subsequently. Repeated imaging and lumbar punctures may be required.
The most sensitive type of imaging for diagnosis of encephalitis is MRI; in HSV encephalitis, CT scans may initially appear normal, but MRI usually shows involvement of the temporal lobes. Electroencephalography is less sensitive, but may be helpful if it shows characteristic features (eg, lateralising periodic sharp and slow wave patterns).
Lumbar puncture usually shows increased opening pressure (> 30 cmH2O), moderate lymphocytic pleocytosis (< 50 × 106 cells/L), and a mild to moderate rise in protein with normal CSF : serum glucose ratio (> 60%). PCR testing of CSF for HSV DNA has high sensitivity and specificity at presentation, but, if negative, should be repeated after three days, especially if red blood cells are present. In encephalitis caused by enteroviruses, PCR and culture of CSF are usually positive for the virus. If an enterovirus is suspected, throat and stool culture should also be performed at presentation.
As diagnostic methods are evolving rapidly, it is important to contact the laboratory and provide sufficient concurrent sera and CSF for the optimum test protocol.
Support in an intensive care unit is often required in encephalitis to maintain ventilation, protect the airway and manage complications, such as cerebral oedema and hypoglycaemia.
HSV encephalitis should be treated with intravenous aciclovir (10 mg/kg three times daily) for two to three weeks,23 or longer in patients who are immunocompromised or have a slow clinical response. Aciclovir-resistant strains have been reported in patients with AIDS. As HSV is a common cause of encephalitis with potentially devastating effects, it is important, in the absence of an alternative diagnosis, to use intravenous aciclovir empirically until results of specific investigations are available.
Varicella–zoster virus encephalitis also requires intravenous aciclovir;24 ganciclovir is more potent but also more toxic. Specific therapies active against enteroviruses (pleconaril) and influenza (neuraminidase inhibitors) have recently become available, but controlled trials have not yet been undertaken in encephalitis.
Routine childhood vaccinations prevent many of the diseases that can cause encephalitis (mumps, poliomyelitis, measles and rubella). Caesarean section and prophylactic aciclovir should be considered in pregnant women with active HSV-2 lesions.
Evidence-based recommendations
Early antibiotic treatment is associated with improved outcome from bacterial meningitis1 (E33).
Dexamethasone should be given to children with meningitis caused by Haemophilus influenzae13 (E1).
Intravenous aciclovir is the treatment of choice for HSV-1 encephalitis12 (E2).
1: Rash in meningococcal meningitis
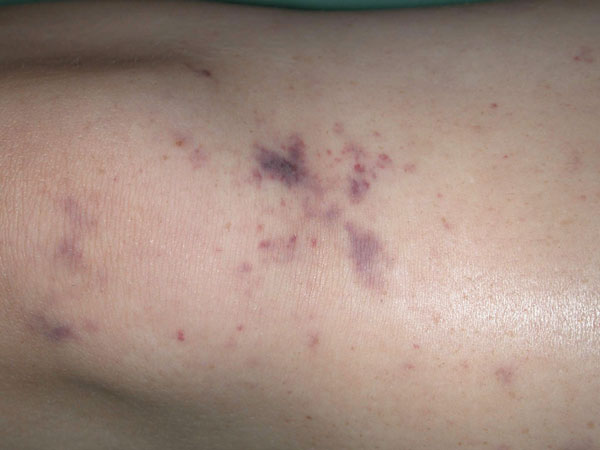
The rash in meningitis caused by Neisseria meningitidis typically has petechial and purpuric components. (Image courtesy of Dr M Hassell, Fremantle Hospital, Fremantle, WA.)
2: Diagnosis and management of acute meningitis and encephalitis
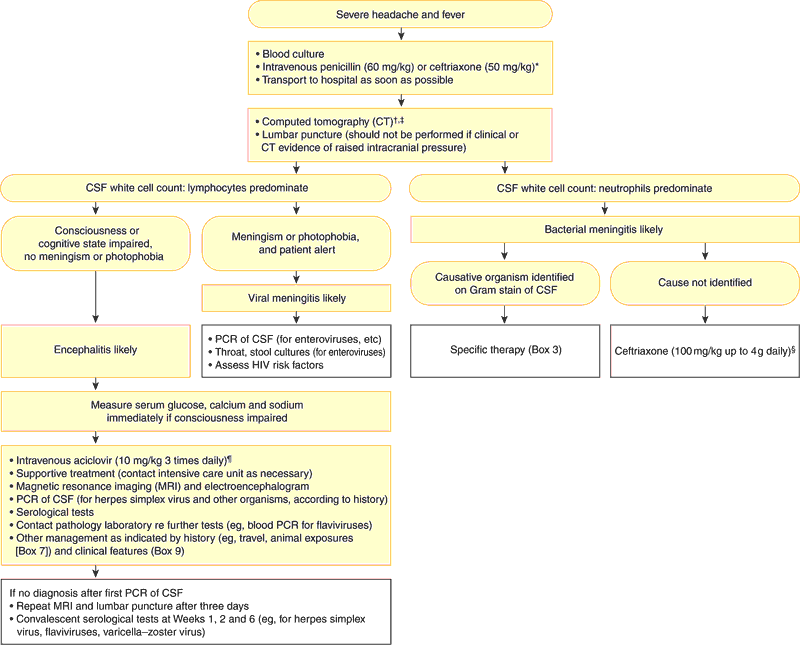
CSF = cerebrospinal fluid. PCR = polymerase chain reaction.
* Antibiotics should be given if there is a rash or if hospital transfer will take more than 30 minutes.
† Empirical antibiotic therapy should be given before CT and lumbar puncture if the patient's condition is unstable.
‡ CT may be judged unnecessary before lumbar puncture if the patient is alert and has no papilloedema or focal neurological signs.
§ If the patient is aged under one month or over 50 years, or is alcoholic, diabetic or receiving corticosteroids, add benzylpenicillin (60 mg/kg up to 1.8 g four-hourly intravenously) to cover the possibility of Listeria infection.
¶ To cover the possibility of herpes simplex virus encephalitis.
3: Typical profiles of cerebrospinal fluid in acute meningitis and encephalitis
Meningitis |
Encephalitis |
||||||||||
Investigation |
Reference range |
Bacterial |
Viral |
||||||||
Opening pressure |
< 30 mmH2O |
Raised |
Normal |
Increased |
|||||||
White cells |
|||||||||||
Total count |
< 5 x 106/L |
Greatly increased |
Moderately increased |
Moderately increased |
|||||||
Differential |
Lymphocytes (60%–70%), monocytes (30%–50%), no neutrophils or red blood cells |
Neutrophils predominate |
Lymphocytes predominate |
Lymphocytes predominate |
|||||||
Glucose concentration |
2.8–4.4 mmol/L |
Decreased |
Normal |
Normal |
|||||||
CSF : serum glucose ratio |
> 60% |
Decreased |
Normal |
Normal |
|||||||
Protein concentration |
< 0.45 g/L |
Increased |
Normal or slightly increased |
Normal or slightly increased |
|||||||
CSF = cerebrospinal fluid. |
|||||||||||
4: Antibiotic treatment of meningitis
Treatment before hospitalisation*
Benzylpenicillin (60 mg/kg, up to) 3 g intravenously or intramuscularly
or ceftriaxone (50 mg/kg, up to) 2 g intravenously (in patients hypersensitive to penicillin or in remote areas where further parenteral therapy may be substantially delayed [over 6 h]).
Empirical treatment in hospital
Cefotaxime (child, 50 mg/kg up to) 2 g intravenously, 6-hourly
or ceftriaxone (child, 100 mg/kg up to) 4 g intravenously, daily in 1 or 2 divided doses for 7 to 10 days
plus benzylpenicillin (child, 60 mg/kg up to) 1.8 g intravenously 4-hourly for 7 to 10 days (if aged under 3 months or over 50 years).
Vancomycin (15 mg/kg up to) 500 mg four times daily intravenously or rifampicin (20 mg/kg up to) 600 mg daily should be added if Streptococcus pneumoniae is suspected on Gram stain, to ensure adequate cover for penicillin- or cephalosporin-intermediate or -resistant isolates before susceptibility results are available.
Specific treament (organism and susceptibility known)
Haemophilus influenzae type b
Cefotaxime (child, 50 mg/kg up to) 2 g intravenously 6-hourly
or ceftriaxone (child, 100 mg/kg up to) 4 g intravenously daily in 1 or 2 divided doses for 7 to 10 days
or, if the organism is proven to be susceptible, (amoxy)ampicillin (child: 50 mg/kg up to) 2 g intravenously, 4-hourly for 7 to 10 days.
Neisseria meningitidis
Benzylpenicillin (child: 60 mg/kg up to) 1.8 g intravenously, 4-hourly for 5 to 7 days.
For patients hypersensitive to penicillin (excluding immediate hypersensitivity), cefotaxime (child: 50 mg/kg up to) 2 g intravenously, 6-hourly or ceftriaxone (child: 100 mg/kg up to) 4 g intravenously daily in 1 or 2 divided doses for 5 to 7 days.
Streptococcus pneumoniae
For strains with minimum inhibitory concentration (MIC) > 0.125 mg/L, vancomycin or rifampicin plus either cefotaxime or ceftriaxone.
For penicillin-susceptible strains (MIC < 0.125 mg/L), benzylpenicillin (child: 60 mg/kg up to) 1.8 g intravenously 4-hourly for at least 10 days.
Listeria monocytogenes
Penicillin and (amoxy)ampicillin appear equally efficacious. In patients hypersensitive to penicillin, trimethoprim–sulfamethoxazole may be used alone.
Trimethoprim–sulfamethoxazole (child: 5/25 mg/kg up to) 160/800 mg intravenously 6-hourly
plus either benzylpenicillin (child: 60 mg/kg up to) 1.8 g intravenously, 4-hourly
or (amoxy)ampicillin (child: 50 mg/kg up to) 2 g intravenously 4-hourly.
* Blood should be collected for culture before antibiotic administration if facilities allow.
5: Case report — meningitis unresponsive to standard empirical therapy
Presentation: A 62-year-old woman recently returned from South Africa presented with a two-day history of headache, photophobia, neck stiffness and fever.
Examination: She had a fever and evidence of meningism but was initially alert.
Investigations: Cerebrospinal fluid (CSF) examination showed white cell count, 100 x 106 cells/L (predominantly neutrophils); protein, 2.4 g/L; glucose, 1.2 mmol/L; CSF : serum glucose ratio, 20%. Computed tomography of the brain gave normal results.
Management: Treatment was begun with ceftriaxone and aciclovir in a peripheral hospital. The patient became increasingly confused over the following 24 hours and was transferred to a tertiary referral centre. Intravenous vancomycin (1 g twice daily) was added to the treatment regimen, but not dexamethasone.
Course and outcome: CSF culture yielded Streptococcus pneumoniae with minimum inhibitory concentrations (MICs) of 4 mg/L for penicillin and 2 mg/L for cefotaxime. Aciclovir therapy was stopped. The patient's condition responded slowly to therapy over the subsequent four days, leaving no long-term sequelae.
Clues suggesting antibiotic-resistant S. pneumoniae as the cause were:
Poor response to initial therapy with ceftriaxone; and
Exposure in a country (South Africa) with high prevalence of multiresistant S. pneumoniae.
6: Chemoprophylaxis for meningitis contacts
Prophylaxis is required for the index case and for all household and close contacts of patients with Haemophilus influenzae and Neisseria meningitidis infection. If in doubt, advice should be sought from public health authorities.
Haemophilus influenzae
Rifampicin (neonate < 1 month, 10 mg/kg; child, 20 mg/kg up to) 600 mg orally, daily for 4 days.
Alternatively, although data are limited, if rifampicin is considered unsuitable, use ceftriaxone (child: 50 mg/kg up to) 1 g intramuscularly, daily for 2 days.
Unvaccinated contacts aged under 5 years should be vaccinated as soon as possible.
Neisseria meningitidis
Rifampicin (neonate < 1 month, 5 mg/kg; child, 10 mg/kg up to) 600 mg orally, 12-hourly for 2 days.
If rifampicin is considered unsuitable, use ceftriaxone 250 mg (child, 125 mg) intramuscularly or ciprofloxacin 500 mg orally, as a single dose.
Non-pregnant adults: Ciprofloxacin 500 mg as a single dose.
7: Causes of encephalitis related to specific exposures
Geographic exposures
Northern Australia: arbovirus infection (Murray Valley, Kunjin),17 scrub typhus; leptospirosis, melioidosis,18 Japanese B encephalitis,19 Hendra virus20 and Australian bat lyssavirus21 infection
North America, Europe: Lyme disease, Ehrlichia infection, Rocky Mountain spotted fever, region-specific arbovirus infections (eg, West Nile, St Louis), rabies, variant Creutzfeldt–Jakob disease
Africa: cerebral malaria, tuberculosis, leptospirosis, typhoid encephalopathy, trypanosomiasis, Borrelia infection, brucellosis
Animal exposures
Bats (Australia): Australian bat lyssavirus
Horses (Australia): Hendra virus
Bites (overseas): rabies (especially bites by canid species in countries with endemic rabies)
Monkeys (overseas, research facilities): herpes B simiae
Cats (Australia and overseas): Bartonella henselae
Rodents (Australia and overseas): murine typhus, hantavirus
Arthropods: arbovirus infection (Murray Valley, Kunjin virus),17 scrub typhus, Q fever
8: Case report — sudden onset of seizures and loss of consciousness
Presentation: A 70-year-old man was taken to a hospital emergency department in a comatose state. He had become ill very suddenly while talking on the telephone — stopping in mid-sentence and having a generalised seizure.
Examination: The patient had a Glasgow Coma Score of 3, which did not respond to intravenous glucose or phenytoin. Initial computed tomography revealed no haemorrhage or focal lesion.
Management: He was admitted to the intensive care unit, ventilated and treated with intravenous aciclovir.
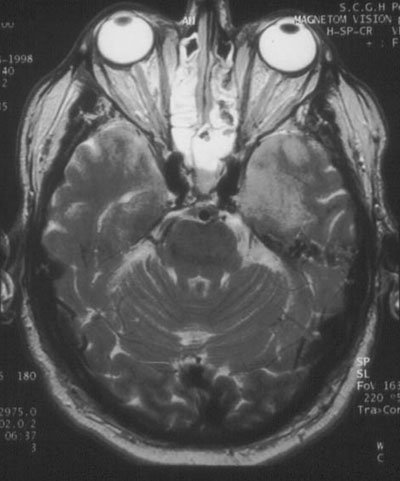
Investigations: Initial analysis of the cerebrospinal fluid (CSF) showed 7 x 106 lymphocytes/L; no red blood cells; protein level, 0.61 g/L; glucose level, 9 mmol/L and CSF : serum ratio, 80%. CSF was negative for cryptococcal antigen and, on polymerase chain reaction testing, for enteroviruses and Murray Valley encephalitis virus, but positive for HSV-1. An electroencephalogram showed periodic lateralising epileptiform discharges in the left temporal lobe. Magnetic resonance imaging on Day 4 showed bilateral temporal lobe abnormalities (Figure).
Diagnosis: Encephalitis caused by herpes simplex virus (HSV).
Course and outcome: Aciclovir treatment was continued, and the patient's condition improved gradually after successful treatment of complications that included aspiration pneumonia. He was transferred from the intensive care unit on Day 21. Although the patient's rehabilitation was complicated by inappropriate behaviour and memory disturbance, he was able to be discharged home on Day 50.
Features suggesting encephalitis were cortical signs (coma and seizure) and cerebrospinal fluid profile (Box 3).
Patients presenting with encephalitic syndromes should receive empirical therapy with aciclovir to cover the possibility of HSV infection until an aetiological cause is identified.
Temporal lobe sequelae (memory disturbance and behavioural abnormality) are characteristic of HSV encephalitis.
9: Clinical clues as to cause of encephalitis
Herpes simplex virus: Temporal lobe signs often prominent (personality change, hallucinations)
Varicella–zoster virus: Cerebellar ataxia (children), progressive confusion (adults)
Epstein–Barr virus: Meningoencephalitis (immunocompromised)
Human herpesvirus 6: Focal encephalitis (immunocompromised adults)
Murray Valley encephalitis virus: Involvement of thalamus, brainstem, cerebellum and spinal cord
Japanese encephalitis virus: Brainstem involvement, meningeal signs may be striking, parkinsonian signs (40% overall mortality)
Cytomegalovirus: Insidiously progressive (similar to AIDS dementia)
Enteroviruses: May occur in epidemics, chronic course in patients with hypogammaglobulinaemia (enterovirus type 71 causes epidemic meningoencephalitis; brainstem involvement prominent)
Poliovirus: Involvement of spinal cord and brainstem
Rabies virus: Hyperaesthesia at inoculation site
Burkholderia pseudomallei (melioidosis): Brainstem involvement
Listeria monocytogenes: Occurs at extremes of age, brainstem involvement
- Miles H Beaman1
- Steven L Wesselingh2
- 1 Department of Infectious Diseases, Fremantle Hospital, Fremantle, WA.
- 2 Infectious Diseases Unit, Alfred Hospital, Melbourne, VIC.
We would like to thank Dr P Tuch for reviewing the manuscript and Dr H Darragh and J McCarthy for helpful discussions (all from Fremantle Hospital, Fremantle, WA).
- 1. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med 1998; 129: 862-869.
- 2. Communicable Diseases Network Australia, Communicable Diseases and Environmental Health Branch, Australian Department of Health and Ageing. Communicable diseases — Australia. Available at <http://www.health.gov.au/internet/wcms/Publishing.nsf/Content/cda-pubs-cdi-cdiintro.htm> Accessed Aug 2005.
- 3. Pfister HW, Feiden W, Einhaupl KM. Spectrum of complications during bacterial meningitis in adults. Results of a prospective clinical study. Arch Neurol 1993; 50: 575-581.
- 4. Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med 1993; 328: 21-28.
- 5. Verghese A, Gallemore G. Kernig's and Brudzinski's signs revisited. Rev Infect Dis 1987; 9: 1187-1192.
- 6. Begg N, Cartwright KAV, Cohen J, et al. Consensus statement on diagnosis, investigation, treatment and prevention of acute bacterial meningitis in immuncompetent adults. J Infect 1999; 39: 1-15.
- 7. Zunt JR, Marra CM. Cerebrospinal fluid testing for the diagnosis of central nervous system infection. Neurol Clin 1999; 17: 675-689.
- 8. Collignon PJ, Turnidge JD. Antibiotic resistance in Streptococcus pneumoniae. Med J Aust 2000; 173 Suppl: S58-S64.
- 9. Hogg GG, Strachan JE, Lester RA. Invasive pneumococcal disease in the population of Victoria. Med J Aust 2000; 173 Suppl: S32-S35.
- 10. Klugman KP, Feldman C. Penicillin- and cephalosporin-resistant Streptococcus pneumoniae: emerging treatment for an emerging problem. Drugs 1999; 58: 1-4.
- 11. Klugman KP, Friedland IR, Bradley JS. Bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis. Antimicrob Agents Chemother 1995; 39: 1988-1992.
- 12. Friedland IR, Paris M, Ehrett S, et al. Evaluation of antimicrobial regimens for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother 1993; 37: 1630-1636.
- 13. McIntyre PB, Berkey CS, King SM, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA 1997; 278: 925-931.
- 14. Roos KL. Encephalitis. Neurol Clin 1999: 17; 813-833.
- 15. Whitley RJ, Lakeman F. Herpes simplex infections of the central nervous system. Clin Infect Dis 1995; 20: 414-420.
- 16. McMinn P, Lindsay K, Perera D, et al. Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia, Singapore and Western Australia. J Virol 2001; 75: 7732-7738.
- 17. Russell RC, Dwyer DE. Arboviruses associated with human disease in Australia. Microbes Infect 2000; 2: 1693-1704.
- 18. Currie BJ, Fisher DA, Howard DM, Burrow JN. Neurological melioidosis. Acta Trop 2000; 74: 145-151.
- 19. Hanna JN, Ritchie SA, Phillips DA, et al. Japanese encephalitis in north Queensland 1998. Med J Aust 1999; 170: 533-536.
- 20. Paterson DL, Murray PK, McCormack JG. Zoonotic disease in Australia caused by a novel member of the paramyxoviridae. Clin Infect Dis 1998; 27: 112-118.
- 21. Allworth A, Murray K, Morgan J. A human case of encephalitis due to a lyssavirus recently identified in fruit bats. Commun Dis Intell 1996; 20: 504.
- 22. Pai H, Sohn S, Seong Y, et al. Central nervous system involvement in patients with scrub typhus. Clin Infect Dis 1997; 24: 436-440.
- 23. Whitley RJ, Alford CA, Hirsch MS, et al. Vidaribine versus aciclovir in Herpes Simplex encephalitis. N Engl J Med 1986; 314: 144-149.
- 24. Whitley RJ, Gnann JW Jr, Hinthorn D, et al. Disseminated herpes zoster in the immunocompromised host. J Infect Dis 1992; 165: 450-455.
- 25. Lyssa Virus Expert Group. Prevention of Lyssa virus infection. Commun Dis Intell 1996; 20: 505-507.





Abstract
Acute meningitis and encephalitis are medical emergencies that require prompt assessment (usually by cerebral imaging and lumbar puncture) and treatment; specialist consultation is recommended.