Acute lower respiratory infections and pneumonia are major causes of morbidity in Indigenous children,1,2 but systematically evaluated data on the burden of these diseases are lacking. Most studies of disease resulting in hospitalisation have relied on hospital discharge diagnosis codes, which may be influenced by changes in clinician practices and coding procedures over time.
In 2001, the World Health Organization published guidelines for the standardised measurement in research of radiologically apparent pneumonia in children.3 These guidelines are now the benchmark on which burden of disease and intervention studies are based.
According to the WHO protocol, radiologically confirmed pneumonia — endpoint consolidation — is defined as “dense opacity . . . fluffy consolidation of a portion or whole of a lobe or of the entire lung, often containing air bronchograms and sometimes associated with pleural effusion”.3 We studied the incidence of hospitalised, radiologically confirmed pneumonia in Northern Territory Indigenous children admitted to hospital over an 8-year period.
In 2001, the NT had an estimated resident population of about 197 800 people4 dispersed across 1 346 200 km2; 29% of the population identify as Aboriginals or Torres Strait Islanders. Two climate zones exist in the NT: tropical in the north (the “Top End”) and arid in the south (the “Centre”). The average annual population of NT resident Indigenous children aged between ≥ 29 days and < 5 years over the study period was 7214 (range, 7047–7382).
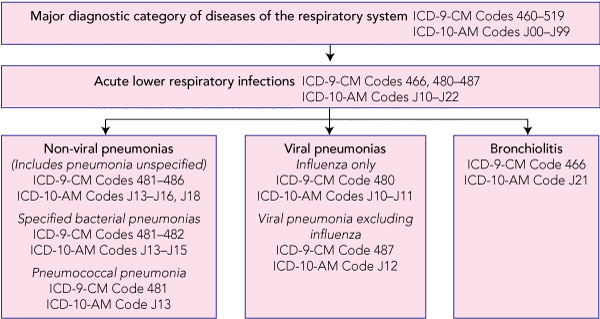
Up to 30 June 1998, the International classification of diseases, 9th revision, clinical modification (ICD-9-CM), was used for morbidity coding of records; for the remaining years, the 10th revision, Australian modification (ICD-10-AM) was used. Box 1 shows the hierarchy of diagnosis codes selected. Unique health record numbers permit linking of data and tracking of individuals in the NT public hospital system.
Of the 24 820 admitted episodes of care, 705 episodes for 295 children and 4541 chest x-rays were excluded from the analysis as they did not meet eligibility criteria (child not an NT resident, x-ray taken ≥ 3 days after admission). The final dataset comprised 24 115 admitted episodes of care for 9492 children and 13 683 x-rays. Box 2 gives the demographic characteristics of the children and the episodes of care.
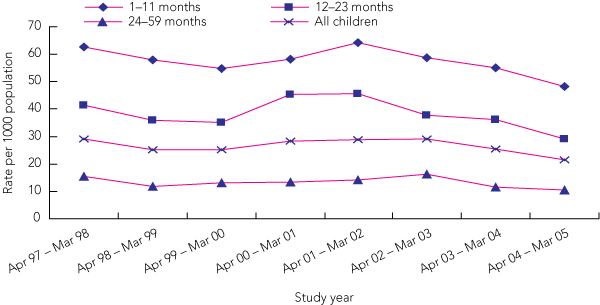
The 1535 episodes of endpoint consolidation occurred in 1211 children with a range of 1–16 episodes per child (one episode, 937 children; two or more episodes, 274 children). Median age at the time of admission was 15 months (range, 1–59 months); 40.8% of episodes were for children aged < 12 months and 23% were for children aged < 6 months. Rates were 1.22 times higher for boys (95% CI, 1.19–1.26; P < 0.001), and 2.1 times higher in children in the southern region of NT (Centre) (95% CI, 1.6–2.8) compared with the Top End (Box 3). The average annual cumulative incidence of endpoint consolidation was 26.6 per 1000 population per year (95% CI, 25.3–27.9); 57.5 per 1000 per year in infants aged 1–11 months, 38.3 per 1000 per year in those aged 12–23 months, and 13.3 per 1000 per year in those aged 24–59 months. Annual rates for each year of the study by age group are shown in Box 4.
Case ascertainment differences may partially explain the high rates of endpoint consolidation we found. First, the entire NT hospitalised population was included, and hospital access has been improving over the past two decades; and, second, we included remote-living children. Comparable data on children in other disadvantaged populations are from studies in predominantly urban or peri-urban populations; children from rural and remote areas, with substantially less access to health services and with differing risk-factor profiles, would have been missed. These studies are from The Gambia (53.0/1000; aged 1–11 months),5 Philippines (13.5/1000; aged 6 weeks to 23 months),6 Indonesia (8.9/1000; aged 1.5–23 months),7 Chile (5.0/1000; aged 4–23 months),8 Fiji (4.3/1000; aged 1–59 months),9 Uruguay (up to 16.9/1000; aged 0–59 months)10 and South Africa (4.9/1000; aged 1.5–30 months).11 Furthermore, we included all chest x-rays taken within the first 3 days of admission. Studies including only x-rays taken on the day of admission may miss some cases, as clinical pneumonia may precede radiologically confirmed endpoint consolidation.
Indigenous children in the NT may have a different risk factor and health care access profile, predisposing them to infection and hospitalisation. Underlying medical conditions or comorbidities (eg, malnutrition, anaemia and gastrointestinal infections) are common,12,13 and the high prevalence of low-birthweight infants is well documented.14-16 Overcrowding, excessive pneumococci and non-typeable Haemophilus influenzae carriage rates in early infancy,17,18 and repeated infections leading to bronchiectasis and chronic lung disease19,20 may play an important role in disease burden.
Measurement error leading to an overestimation of disease incidence is unlikely. All readers were blinded to the clinical diagnosis associated with each chest x-ray film. While readers might have been influenced by knowing that they were examining x-rays from a high-risk population, the incidence of endpoint consolidation found was lower than that anticipated from a priori estimates based on data from Central Australia.21 Furthermore, the proportion of admitted episodes of care deemed positive for endpoint consolidation (20% of all episodes of ALRI in which a chest x-ray was taken) was similar to that reported in other studies.5,11,22
- Kerry-Ann F O’Grady1,2
- Debbie M Taylor-Thomson1
- Anne B Chang1
- Paul J Torzillo4
- Peter S Morris1,5
- Grant A Mackenzie6
- Gavin R Wheaton7
- Paul A Bauert8
- Margaret P De Campo9
- John F De Campo9
- Alan R Ruben5
- 1 Menzies School of Health Research, Charles Darwin University, Darwin, NT.
- 2 School of Population Health and Department of Paediatrics, University of Melbourne, Melbourne, VIC.
- 3 Vaccine and Immunisation Research Group, Murdoch Childrens Research Institute, Melbourne, VIC.
- 4 Royal Prince Alfred Hospital, University of Sydney, Sydney, NSW.
- 5 Northern Territory Clinical School, Flinders University, Darwin, NT.
- 6 Bacterial Diseases Program, Medical Research Council (UK) Laboratories, Fajara, The Gambia.
- 7 Department of Clinical Effectiveness, School of Medicine, Faculty of Health Sciences, Flinders University, Adelaide, SA.
- 8 Royal Darwin Hospital, Darwin, NT.
- 9 Bond University, Gold Coast, QLD.
We thank Ross Andrews, Joan Cunningham (Menzies School of Health Research); Terry Nolan (University of Melbourne); John Carlin, Suzanna Vidmar (Murdoch Childrens Research Institute); Jane Benson (Johns Hopkins University Hospital); and Kim Mulholland, Tilman Ruff, and Thomas Cherian.
Wyeth Vaccines provided funding for the study, but had no role in the design, data collection, analysis and interpretation, writing, or publication of the article. Kerry-Ann O’Grady has been a senior research officer on sponsored vaccine trials (GlaxoSmithKline, Wyeth, Merck Sharp & Dohme, MedImmune, and CSL) and a recipient of funds for epidemiological research (GlaxoSmithKline).
- 1. Carville KS, Lehmann D, Hall G, et al. Infection is the major component of the disease burden in Aboriginal and non-Aboriginal Australian children: a population-based study. Pediatr Infect Dis J 2007; 26: 210-216.
- 2. Burgner D, Richmond P. The burden of pneumonia in children: an Australian perspective. Paediatr Respir Rev 2005; 6: 94-100.
- 3. World Health Organization Pneumonia Vaccine Trial Investigators Group. Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. Geneva: World Health Organization Department of Vaccines and Biologicals, 2001. http://www.who.int/vaccines-documents/DocsPDF01/www616.pdf (accessed Mar 2010).
- 4. Australian Bureau of Statistics. Census of population and housing: selected social and housing characteristics for Statistical Local Areas, Northern Territory, 2001. (ABS Cat. No. 2015.7.)
- 5. Cutts FT, Zaman SM, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 2005; 365: 1139-1146.
- 6. Lucero MG, Nohynek H, Williams G, et al. Efficacy of an 11-valent pneumococcal conjugate vaccine against radiologically confirmed pneumonia among children less than 2 years of age in the Philippines: a randomized, double-blind, placebo-controlled trial. Pediatr Infect Dis J 2009; 28: 455-462.
- 7. Gessner BD, Sutanto A, Linehan M, et al. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: hamlet-randomised vaccine-probe trial. Lancet 2005; 365: 43-52.
- 8. Levine OS, Lagos R, Munoz A, et al. Defining the burden of pneumonia in children preventable by vaccination against Haemophilus influenzae type b. Pediatr Infect Dis J 1999; 18: 1060-1064.
- 9. Magree HC, Russell FM, Sa’aga R, et al. Chest x-ray confirmed pneumonia in children in Fiji. Bull World Health Organ 2005; 83: 427-434.
- 10. Hortal M, Estevan M, Iraola I, De Mucio B. A population-based assessment of the disease burden of consolidated pneumonia in hospitalized children under five years of age. Int J Infect Dis 2007; 11: 273-277.
- 11. Klugman KP, Madhi SA, Heubner R, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 2003; 349: 1341-1348.
- 12. Paterson B, Ruben A, Nossar V. School screening in remote Aboriginal communities — results of an evaluation. Aust N Z J Public Health 1998; 22: 685-689.
- 13. Ruben AR, Walker AC. Malnutrition among rural Aboriginal children in the Top End of the Northern Territory. Med J Aust 1995; 162: 400-403.
- 14. Mackerras DE, Reid A, Sayers SM, et al. Growth and morbidity in children in the Aboriginal Birth Cohort Study: the urban–remote differential. Med J Aust 2003; 178: 56-60. <MJA full text>
- 15. Gogna NK, Smiley M, Walker AC, Fullerton P. Low birthweight and mortality in Australian Aboriginal babies at the Royal Darwin Hospital: a 15-year study. Aust Paediatr J 1986; 22: 281-284.
- 16. Rousham EK, Gracey M. Factors affecting birthweight of rural Australian Aborigines. Ann Hum Biol 2002; 29: 363-372.
- 17. Leach AJ, Boswell JB, Asche V, et al. Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian Aboriginal infants. Pediatr Infect Dis J 1994; 13: 983-989.
- 18. Smith-Vaughan HC, Leach AJ, Shelby-James TM, et al. Carriage of multiple ribotypes of non-encapsulated Haemophilus influenzae in Aboriginal infants with otitis media. Epidemiol Infect 1996; 116: 177-183.
- 19. Valery PC, Torzillo PJ, Mulholland K, et al. Hospital-based case–control study of bronchiectasis in Indigenous children in Central Australia. Pediatr Infect Dis J 2004; 23: 902-908.
- 20. Chang AB, Masel JP, Boyce NC, Torzillo PJ. Respiratory morbidity in central Australian Aboriginal children with alveolar lobar abnormalities. Med J Aust 2003; 178: 490-494. <MJA full text>
- 21. Torzillo P, Dixon J, Manning K, et al. Etiology of acute lower respiratory tract infection in Central Australian Aboriginal children. Pediatr Infect Dis J 1999; 18: 714-721.
- 22. Hansen J, Black S, Shinefield H, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J 2006; 25: 779-781.





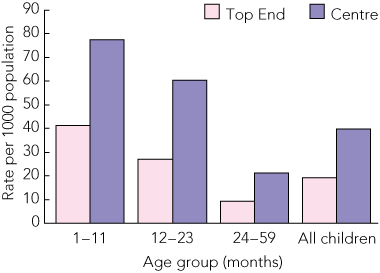
Abstract
Objective: To determine the burden of hospitalised, radiologically confirmed pneumonia (World Health Organization protocol) in Northern Territory Indigenous children.
Design, setting and participants: Historical, observational study of all hospital admissions for any diagnosis of NT resident Indigenous children, aged between ≥ 29 days and < 5 years, 1 April 1997 to 31 March 2005.
Intervention: All chest radiographs taken during these admissions, regardless of diagnosis, were assessed for pneumonia in accordance with the WHO protocol.
Main outcome measure: The primary outcome was endpoint consolidation (dense fluffy consolidation [alveolar infiltrate] of a portion of a lobe or the entire lung) present on a chest radiograph within 3 days of hospitalisation.
Results: We analysed data on 24 115 hospitalised episodes of care for 9492 children and 13 683 chest radiographs. The average annual cumulative incidence of endpoint consolidation was 26.6 per 1000 population per year (95% CI, 25.3–27.9); 57.5 per 1000 per year in infants aged 1–11 months, 38.3 per 1000 per year in those aged 12–23 months, and 13.3 per 1000 per year in those aged 24–59 months. In all age groups, rates of endpoint consolidation in children in the arid southern region of NT were about twice that of children in the tropical northern region.
Conclusion: The rates of severe pneumonia in hospitalised NT Indigenous children are among the highest reported in the world. Reducing this unacceptable burden of disease should be a national health priority.