Otitis media is a common disease that affects many children and their families across the globe. The burden of otitis media and the frequency and severity of infection varies among populations. In developed countries, acute otitis media (AOM) is the most common reason for antibiotic prescription, with possible links to rising rates of antibiotic-resistant bacterial pathogens. Estimates of the incidence of AOM from longitudinal birth cohort studies in developed countries range from 0.125 to 1.2 episodes per child-year.1-3
Australian Indigenous children experience a mean of five episodes of AOM or chronic suppurative otitis media (CSOM) per child-year.4 In a Far North Queensland paediatric outreach program, CSOM was identified as the most common health problem among Aboriginal children.5 A 2005 survey of 644 children aged 6 months to 2.5 years conducted by trained research personnel across 17 remote Northern Territory communities found that 20% of children had tympanic membrane perforation, and around 12% had bilaterally normal ears.6
The main pathogens associated with otitis media are Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis. We hypothesise that increased bacterial load in the nasopharynx is associated with perforation; this is supported by our recent study, which showed a greater probability of suppurative otitis media (AOM with or without perforation) as density of S. pneumoniae, H. influenzae and M. catarrhalis increases.7 The probability of suppurative otitis media increased from 20% at a pathogen density of less than 105/mL, to 40% at a pathogen density of 106/mL, to 50% at a pathogen density of 107/mL.7 A much lower probability (10%) was associated with total bacterial density (commensal and pathogen density combined) of 106/mL. Alloiococcus otitidis has been implicated by culture or polymerase chain reaction (PCR) in chronic otitis media with effusion (OME) in several studies, including one of Australian Indigenous and non-Indigenous children undergoing myringotomy.8 The proportion of middle ear fluids positive for A. otitidis was far greater in these children than all other reported studies — cultures from 10 of 22 Indigenous and 10 of 28 non-Indigenous children tested positive.
The specific risk factors that lead to an increased density of otitis media pathogens are not known, but it is plausible that antecedent viral infection plays a role. The availability of improved detection methods has provided opportunities for exploring the role of respiratory viruses in otitis media.9 In most regions and populations, winter peaks of AOM typically follow viral (particularly respiratory syncytial virus) epidemics,10 but in the tropical regions of the NT, seasonality of AOM is not observed. Current studies are further investigating the role of viruses in otitis media and the potential effect of influenza vaccination11 on otitis media prevention.
In a Western Australian study, the proportion of specimens positive for otitis media pathogens was higher among Aboriginal children than non-Aboriginal children (S. pneumoniae, 50% v 28%; H. influenzae, 42% v 13%; M. catarrhalis, 52% v 30%; adenovirus, 9% v 4%; and rhinovirus, 23% v 17%).12 Rhinovirus infection was positively correlated with carriage of S. pneumoniae, H. influenzae and M. catarrhalis, and adenovirus with M. catarrhalis. The increased frequency of severe otitis media among Indigenous children could be related to greater frequency of interaction between viral and bacterial pathogens.
Two large studies from Israel and Finland reported data on culture of middle ear fluid obtained by tympanocentesis at the time of acute infection. Both studies found that S. pneumoniae, non-typeable H. influenzae and M. catarrhalis were the commonest causes of AOM.13,14 Interestingly, when Indigenous children in the NT are screened for middle ear infections, around 20% have AOM (with a bulging tympanic membrane) in the absence of symptoms (pain) or fever (Associate Professor Peter Morris, Deputy Leader, Child Health, Menzies School of Health Research, personal communication). In cases of AOM with perforation in this population (tympanocentesis has not been possible), H. influenzae could be cultured from 57% of ear discharge cultures, S. pneumoniae from 34% and M. catarrhalis from less than 10%.15
Up to 73% of acute episodes of otitis media resolve clinically without antibiotic treatment, with no remaining pain and fever (middle ear effusion may persist), within 24 to 48 hours.16 Asymptomatic OME may persist for several weeks (63%) or months (26% after 3 months) after an episode of AOM.16 It is not known whether OME is an infectious process in itself or whether it is residual sterile fluid following AOM. The proportion of middle ear effusions of OME patients that are positive for pneumococci after PCR analysis (13%) has been found to be higher than after conventional culture (5%), but is still small. Antibiotic treatment of OME reduces the proportion of children with persistent OME by about 15% (95% CI, 7%–23%) at median time of 4 weeks.16 In contrast, interventions such as antihistamines and decongestants do not effectively aid OME resolution (risk difference, 0; 95% CI, 7–7). This supports the hypothesis that OME is, in part, a bacterial infection.
AOM with perforation generally requires a longer period of antibiotic therapy to resolve symptoms than AOM without perforation. Recurrent perforations may lead to CSOM (persistent discharge of at least 2–6 weeks) and a larger perforation size. The organisms involved in CSOM are dominantly opportunistic pathogens, particularly Pseudomonas aeruginosa. In most countries where studies have been conducted, P. aeruginosa is the predominant organism and is associated with around 20% to 50% of cases of CSOM; Staphylococcus aureus is also commonly isolated, but proportions of samples positive for S. aureus differ from study to study.17-21 Among Aboriginal children, CSOM is also associated with non-typeable H. influenzae (22%), whereas S. pneumoniae is rarely cultured (3%).17 Studies are needed to evaluate whether systemic antibiotics should be recommended in addition to topical antibiotics for young children with CSOM.
Among Australian Indigenous children, respiratory tract infections commence very early in life and involve a high density of multiple bacterial species and strains; these factors predict recurrent episodes of AOM and progression to CSOM.7,22
Indigenous infants whose nasopharynges were colonised with mixed infections and those involving S. pneumoniae and H. influenzae were at greater risk of otitis media (odds ratio, 33.6; 95% CI, 8–144) than those colonised with M. catarrhalis alone (odds ratio, 6.5; 95% CI, 2–29).22 A study of cultures of ear discharge from acute perforations supports this mixed bacterial aetiology; 57% of cultures yielded H. influenzae, 34% contained S. pneumoniae and 21% yielded both pathogens.15
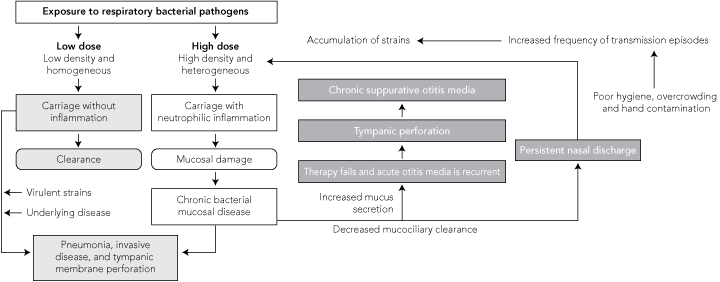
A vicious circle model of inflammation was developed to explain the pathogenesis of chronic suppurative lung disease in adults with bronchiectasis.23 An extension of this model has been proposed to explain the high rates of otitis media and other respiratory infections, such as bronchiectasis, among Indigenous children (Box).24,25 The density of these pathogens, their dominance among the nasopharyngeal flora, and the multiplicity of strains colonising simultaneously escalate during the first weeks of life7 and are all significantly associated with the presence and severity of current ear disease (no otitis media, OME, or suppurative otitis media).26 A comparison of Aboriginal and non-Aboriginal children, all with a diagnosis of OME, demonstrated significantly higher nasopharyngeal loads of S. pneumoniae and M. catarrhalis in the Aboriginal group.7 The increased bacterial load, despite similar clinical condition, could predict persistence of middle ear effusions and progression to suppurative otitis media in the Aboriginal population. Among adults, this dense and diverse colonisation causes neutrophilic infiltration, tissue damage, increased mucus secretion, and decreased mucociliary clearance, leading to chronic suppurative lung disease.23 However, among infants, this cycle may also lead to CSOM and persistent nasal discharge.
The persistent nasal discharge perpetuates the vicious cycle, particularly where there is poor hygiene and overcrowding, by increasing opportunities for transmission among infants. This includes transmission via hand contamination (in a community-based survey, 40% of children were found to have pneumococcal hand contamination).27 High rates of transmission events result in an accumulation of strains at a rate greater than can be cleared by the infant immune response or by damaged mucosa, and the vicious circle is repeated. For the middle ear, high density of organisms and damaged mucosa are also likely risk factors for failed therapy, recurrent AOM, and progression to perforation and CSOM.
Environmental risk factors for otitis media include season, respiratory viral infection, exposure to other children, environmental tobacco smoke exposure, lack of breastfeeding and dummy (pacifier) use. Most of these risk factors enhance opportunities for upper respiratory tract and nasopharyngeal colonisation by otitis media bacterial pathogens through increased exposure to multiple strains (from multiple children), increased transmission (due to crowding and aerosol spread in winter and during viral outbreaks), reduced clearance (from reduced mucociliary function due to environmental tobacco smoke, poor hygiene and lack of passive immunity). Swimming pools in remote communities, where prepool prevalence of infections was high, are associated with reduced rates of antibiotic prescription (45%), clinic attendance for middle ear infections (61%) and respiratory tract infections (52%).28 Swimming pools may reduce these infections via chlorination or through more thorough physical removal of bacterial load in sores or the nasopharynx. Whether swimming in the absence of chlorination or increased frequency and duration of showering in the home would achieve similar reductions in otitis media is not clear, but this could be evaluated in appropriately designed trials.
In addition, several host risk factors contribute to the development of otitis media, one of these being young age. Most children (50%–85%) experience an otitis media episode in the first 3 years of life, with a peak incidence between 6 and 11 months of age.2 This peak tends to coincide with the transition from breastfeeding and increased exposure to other children — either directly through daycare attendance or because of overcrowding. For Aboriginal children, disease starts early in life — within weeks of birth — and coincides with acquisition of otitis media pathogens.22 Several birth cohort studies in different populations have shown that early age of nasopharyngeal acquisition of respiratory and otitis media pathogens was associated with increased risk of disease.29-34 The mean age of pneumococcal acquisition was close to 30 days in the Gambia34 and Papua New Guinea,30 60 days in Kilifi, Kenya,29and among Australian Indigenous children in the NT,22 6 months in Alabama33 and 9 to 10 months in Costa Rica31 and Buffalo, New York.35
Twin studies36 and the fact that otitis media is more common in certain populations, such as Aboriginal Australian, Inuit and Native American people,37,38 suggest that genetic factors play a role in otitis media susceptibility. Exactly which genes are involved in otitis media susceptibility needs to be established.
Bacterial nasopharyngeal carriage in early infancy, when the immune system is still immature, is associated with subsequent recurrent and/or persistent infections, with relative risks depending on host and environmental risk factors.39 Early bacterial carriage may also affect the development of long-term humoral and cellular immunity, resulting in less effective pathogen clearance. In addition, recurrent infections are more likely to occur because of a deficient innate and/or adaptive immune response.
The innate immune system is an evolutionary, ancient form of immunity, and offers the main resistance to microbial pathogens within the first minutes, hours or days of an infection. Innate immune responses provide critical defence in early life, as passive maternal antibody wanes and there is relative immaturity of adaptive immune responses. There might be a role for subtle deficiencies in innate immune responses in children with recurrent otitis media. Although there are publications on specific innate immune factors,40 toll-like-receptors,41,42 mannose-binding lectin43-46 and soluble CD14,47,48 further research is needed to elucidate which innate immune mechanisms are the most important, and how these innate systems interact and contribute to otitis media susceptibility.
Most data on the natural development of specific immunity against pneumococci and otitis media have focused on serum IgG antibodies against pneumococcal polysaccharides. Pneumococcal polysaccharide-specific mucosal IgA and serum IgG antibodies gradually increase in early childhood following exposure through carriage of the relevant serotypes.49 IgG antibodies in serum appear to protect against the development of otitis media, but do not reduce nasopharyngeal carriage. Serotype-specific mucosal IgA antibodies reduce colonisation by the particular serotype; however, these antibodies do not protect against subsequent colonisation with other bacterial strains or serotypes. It is possible that children with recurrent AOM might produce serotype- and strain-specific antibodies, but fail to develop a broadly protective antibody response against conserved protein antigens. This subtle immunodeficiency might be a mechanism that makes certain children more susceptible to otitis media.50
In addition to antibody production, results of recent studies have suggested that CD4+ T-cell mediated mechanisms are required for optimal production of antibodies against pneumococcal proteins, for development of immunity to pneumococcal colonisation and for protection against mucosal infections due to non-typeable H. influenzae in murine models.51-54 However, such studies have not examined high-risk populations, including children with recurrent otitis media, and it is not known whether these T-cell responses are altered in children with recurrent AOM.
For Indigenous children and others at increased risk of early age of otitis media onset and progression to tympanic membrane perforation, strategies to reduce exposure to high doses of multiple otitis media pathogens are urgently needed. Reduced crowding, improved household facilities for washing children and increased awareness of bacterial transmission among family members could contribute to delayed onset of otitis media. Maternal immunisation and vaccines with expanded bacterial protection and that can be commenced earlier in life are potential strategies. Long-term antibiotic therapy reduces the proportion of infants experiencing tympanic membrane perforation,4 but these schedules are difficult for families. Alternative antibiotic regimens include single-dose azithromycin, which is not superior in resolving AOM compared with 7 days of amoxycillin, but is easier to administer.55 Although there are some general indications that Indigenous child health has improved, such as increased birthweight and lower infant mortality, there is evidence to suggest that morbidity associated with infections, such as otitis media, has not changed over the past 30 years.56
- Selma P Wiertsema1
- Amanda J Leach2
- 1 School of Paediatrics and Child Health, University of Western Australia, Perth, WA.
- 2 Child Health, Menzies School of Health Research, Darwin, NT.
None identified.
- 1. Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 2006; 367: 740-748.
- 2. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis 1989; 160: 83-94.
- 3. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001; 344: 403-409.
- 4. Leach AJ, Morris PS, Mathews JD. Compared with placebo, long-term antibiotics resolve otitis media with effusion (OME) and prevent acute otitis media with perforation (AOMwiP) in a high-risk population: a randomized controlled trial. BMC Pediatr 2008; 8: 23.
- 5. Rothstein J, Heazlewood R, Fraser M. Health of Aboriginal and Torres Strait Islander children in remote Far North Queensland: findings of the Paediatric Outreach Service. Med J Aust 2007; 186: 519-521. <MJA full text>
- 6. Morris PS, Leach AJ, Silberberg P, et al. Otitis media in young Aboriginal children from remote communities in northern and central Australia: a cross-sectional survey. BMC Pediatr 2005; 5: 27.
- 7. Smith-Vaughan H, Byun R, Nadkarni M, et al. Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord 2006; 6: 10.
- 8. Ashhurst-Smith C, Hall ST, Walker P, et al. Isolation of Alloiococcus otitidis from Indigenous and non-Indigenous Australian children with chronic otitis media with effusion. FEMS Immunol Med Microbiol 2007; 51: 163-170.
- 9. Nokso-Koivisto J, Raty R, Blomqvist S, et al. Presence of specific viruses in the middle ear fluids and respiratory secretions of young children with acute otitis media. J Med Virol 2004; 72: 241-248.
- 10. Henderson FW, Collier AM, Sanyal MA, et al. A longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusion. N Engl J Med 1982; 306: 1377-1383.
- 11. Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359: 1555-1564.
- 12. Jacoby P, Watson K, Bowman J, et al. Modelling the co-occurrence of Streptococcus pneumoniae with other bacterial and viral pathogens in the upper respiratory tract. Vaccine 2006; 25: 2458-2464.
- 13. Palmu AA, Herva E, Savolainen H, et al. Association of clinical signs and symptoms with bacterial findings in acute otitis media. Clin Infect Dis 2004; 38: 234-242.
- 14. Leibovitz E, Serebro M, Givon-Lavi N. Epidemiologic and microbiologic characteristics of culture-positive spontaneous otorrhea in children with acute otitis media. Pediatr Infect Dis J 2009; 28: 381-384.
- 15. Leach A. Microbiology of otitis media with perforation in Indigenous children, streptococci — new insights into an old enemy. In: Kadaba E, Sriprakash S, editors. Proceedings of the XVIth Lancefield International Symposium on Streptococci and Streptococcal Diseases; 2006 Sep 25–29; Cairns, Qld: 89-92.
- 16. Rosenfeld R, Bluestone C, editors. Evidence based otitis media. 2nd ed. London: BC Decker, 2003.
- 17. Leach A, Wood Y, Gadil E, et al. Topical ciprofloxin versus topical framycetin–gramicidin–dexamethasone in Australian Aboriginal children with recently treated chronic suppurative otitis media: a randomized controlled trial. Pediatr Infect Dis J 2008; 27: 692-698.
- 18. Altuntas A, Aslan A, Eren N, et al. Susceptibility of microorganisms isolated from chronic suppurative otitis media to ciprofloxacin. Eur Arch Otorhinolaryngol 1996; 253: 364-366.
- 19. Nyembue DT, Tshiswaka JM, Sabue MJ, Muyunga CK. Bacteriology of chronic suppurative otitis media in Congolese children. Acta Otorhinolaryngol Belg 2003; 57: 205-208.
- 20. Sharma S, Rehan HS, Goyal A, et al. Bacteriological profile in chronic suppurative otitis media in Eastern Nepal. Trop Doct 2004; 34: 102-104.
- 21. Aslam MA, Ahmed Z, Azim R. Microbiology and drug sensitivity patterns of chronic suppurative otitis media. J Coll Physicians Surg Pak 2004; 14: 459-461.
- 22. Leach AJ, Boswell JB, Asche V, et al. Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian Aboriginal infants. Pediatr Infect Dis J 1994; 13: 983-989.
- 23. Cole PJ. Inflammation: a two-edged sword — the model of bronchiectasis. Eur J Respir Dis Suppl 1986; 147: 6-15.
- 24. Leach AJ, Morris PS. Perspectives on infective ear disease in Indigenous Australian children. J Paediatr Child Health 2001; 37: 529-530.
- 25. Chang AB, Grimwood K, Mulholland EK, Torzillo PJ. Bronchiectasis in Indigenous children in remote Australian communities. Med J Aust 2002; 177: 200-204. <MJA full text>
- 26. Smith-Vaughan H, Byun R, Halpin S, et al. Interventions for prevention of otitis media may be most effective if implemented in the first weeks of life. Int J Pediatr Otorhinolaryngol 2008; 72: 57-61.
- 27. Stubbs E, Hare K, Wilson C, et al. Streptococcus pneumoniae and noncapsular Haemophilus influenzae nasal carriage and hand contamination in children: a comparison of two populations at risk of otitis media. Pediatr Infect Dis J 2005; 24: 423-428.
- 28. Silva DT, Lehmann D, Tennant MT, et al. Effect of swimming pools on antibiotic use and clinic attendance for infections in two Aboriginal communities in Western Australia. Med J Aust 2008; 188: 594-598. <MJA full text>
- 29. Abdullahi O, Nyiro J, Lewa P, et al. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Pediatr Infect Dis J 2008; 27: 59-64.
- 30. Gratten M, Gratten H, Poli A, et al. Colonisation of Haemophilus influenzae and Streptococcus pneumoniae in the upper respiratory tract of neonates in Papua New Guinea: primary acquisition, duration of carriage, and relationship to carriage in mothers. Biol Neonate 1986; 50: 114-120.
- 31. Vives M, Garcia ME, Saenz P, et al. Nasopharyngeal colonization in Costa Rican children during the first year of life. Pediatr Infect Dis J 1997; 16: 852-858.
- 32. Faden H, Duffy L, Wasielewski R, et al. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J Infect Dis 1997; 175: 1440-1445.
- 33. Gray BM, Converse GM 3rd, Dillon HC Jr. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis 1980; 142: 923-933.
- 34. Hill PC, Cheung YB, Akisanya A, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian infants: a longitudinal study. Clin Infect Dis 2008; 46: 807-814.
- 35. Faden H, Waz MJ, Bernstein JM, et al. Nasopharyngeal flora in the first three years of life in normal and otitis-prone children. Ann Otol Rhinol Laryngol 1991; 100: 612-615.
- 36. Casselbrant ML, Mandel EM, Fall PA, et al. The heritability of otitis media: a twin and triplet study. JAMA 1999; 282: 2125-2130.
- 37. Curns AT, Holman RC, Shay DK, et al. Outpatient and hospital visits associated with otitis media among American Indian and Alaska native children younger than 5 years. Pediatrics 2002; 109: E41.
- 38. Leach AJ. Otitis media in Australian Aboriginal children: an overview. Int J Pediatr Otorhinolaryngol 1999; 49 Suppl 1: S173-S178.
- 39. García-Rodríguez JA, Fresnadillo Martínez MJ. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother 2002; 50 Suppl S2: 59-73.
- 40. Lee HY, Andalibi A, Webster P, et al. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect Dis 2004; 4: 12.
- 41. Song JJ, Cho JG, Woo JS, et al. Differential expression of toll-like receptors 2 and 4 in rat middle ear. Int J Pediatr Otorhinolaryngol 2009; 73: 821-824.
- 42. Hernandez M, Leichtle A, Pak K, et al. Myeloid differentiation primary response gene 88 is required for the resolution of otitis media. J Infect Dis 2008; 198: 1862-1869.
- 43. Hirano T, Kodama S, Fujita K, et al. Role of toll-like receptor 4 in innate immune responses in a mouse model of acute otitis media. FEMS Immunol Med Microbiol 2007; 49: 75-83.
- 44. Wiertsema SP, Herpers BL, Veenhoven RH, et al. Functional polymorphisms in the mannan-binding lectin 2 gene: effect on MBL levels and otitis media. J Allergy Clin Immunol 2006; 117: 1344-1350.
- 45. Homoe P, Madsen HO, Sandvej K, et al. Lack of association between mannose-binding lectin, acute otitis media and early Epstein–Barr virus infection among children in Greenland. Scand J Infect Dis 1999; 31: 363-366.
- 46. Straetemans M, Wiertsema SP, Sanders EA, et al. Immunological status in the aetiology of recurrent otitis media with effusion: serum immunoglobulin levels, functional mannose-binding lectin and Fc receptor polymorphisms for IgG. J Clin Immunol 2005; 25: 78-86.
- 47. Wiertsema SP, Khoo S-K, Baynam G, et al. Association of CD14 promoter polymorphism with otitis media and pneumococcal vaccine responses. Clin Vaccine Immunol 2006; 13: 892-897.
- 48. Lodrup Carlsen KC, Granum B. Soluble CD14: role in atopic disease and recurrent infections, including otitis media. Curr Allergy Asthma Rep 2007; 7: 436-443.
- 49. Balmer P, Borrow R, Findlow J, et al. Age-stratified prevalences of pneumococcal-serotype-specific immunoglobulin G in England and their relationship to the serotype-specific incidence of invasive pneumococcal disease before the introduction of the pneumococcal 7-valent conjugate vaccine. Clin Vaccine Immunol 2007; 14: 1442-1450.
- 50. Faden H. The microbiologic and immunologic basis for recurrent otitis media in children. Eur J Pediatr 2001; 160: 407-413.
- 51. Zhang Q, Bernatoniene J, Bagrade L, et al. Serum and mucosal antibody responses to pneumococcal protein antigens in children: relationships with carriage status. Eur J Immunol 2006; 36: 46-57.
- 52. Simell B, Kilpi T, Kayhty H. Subclass distribution of natural salivary IgA antibodies against pneumococcal capsular polysaccharide of type 14 and pneumococcal surface adhesin A (PsaA) in children. Clin Exp Immunol 2006; 143: 543-549.
- 53. McMahon M, Murphy TF, Kyd J, Thanavala Y. Role of an immunodominant T cell epitope of the P6 protein of nontypeable Haemophilus influenzae in murine protective immunity. Vaccine 2005; 23: 3590-3596.
- 54. Malley R, Srivastava A, Lipsitch M, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun 2006; 74: 2187-2195.
- 55. Morris PS, Gadil JR, McCallum GB, et al. Single-dose azithromycin versus 7 days amoxycillin in the treatment of acute otitis media in Aboriginal children (AATAAC): a double blind, randomised controlled trial. Med J Aust. In press.
- 56. Moran DJ, Waterford JE, Hollows F, Jones DL. Ear disease in rural Australia. Med J Aust 1979; 2: 210-212.





Abstract
Otitis media is a common childhood illness associated with hearing loss, social disadvantage and medical costs. Prevalence and severity are high among Indigenous children.
Respiratory bacterial and viral pathogens ascend the eustachian tube from the nasopharynx to the middle ear, causing inflammation, fluid accumulation, and bulging of the tympanic membrane, with or without pain.
Among Australian Indigenous children, ear disease commences earlier in life, and involves multiple strains of bacterial pathogens at high density that persist longer.
Persistent nasal discharge, overcrowded living conditions (particularly exposure to many children) and poor facilities for washing children perpetuate a vicious cycle of transmission and infection.
Risk factors include environmental tobacco smoke, season, lack of breastfeeding, younger age and immature immune system, and possibly genetic factors.
The innate immune system is a critical first response to infection, particularly as passive maternal antibodies decline and during the maturation of the infant adaptive immune response. The relative contributions of innate factors to protection from otitis media are currently not well understood.
A diversity of antibodies that target strain-specific and conserved antigens are generated in response to natural exposure to otitis media pathogens (or to vaccines). Deficiencies in these antibodies may explain susceptibility to recurrent infections.
Incremental contributions from all these elements are likely to be important in otitis media susceptibility versus protection.
Effective medical and social strategies to prevent early age of onset are urgently needed.