Aboriginal children are seriously disadvantaged and suffer very high rates of infections.1-4 In Western Australia, hospital admission rates for children under the age of 2 years for any infection, for skin infections and for pneumonia are, respectively, five, 12 and 13 times higher in Aboriginal than non-Aboriginal children.2 Prevalence as high as 91% for otitis media and 70% for pyoderma have been documented in remote Aboriginal communities.5-7 Of further concern is the steady increase in Australia in the resistance of respiratory pathogens to antibiotics, making infections more difficult to treat.8
In 2000, swimming pools were installed in four remote Aboriginal communities in WA, with the aim of reducing the burden of disease and to provide social and recreational opportunities for children. The Royal Life Saving Society managed all the pools and ensured standardised testing of water quality, supervision, and regulations on access to the pools, including showering before entering the pool, exclusion of people with certain medical conditions (eg, gastroenteritis), and limiting the number of people using the pool at any one time. Six-monthly surveys in two of the communities before and after installation of the pools showed a reduction in the prevalence of pyoderma and otitis media.6
Swimming pools were opened in Jigalong and Mugarinya in September 2000 and have generally been open from September to April each year. Both communities are in a semi-arid environment, over 1200 km north of Perth (Box 1). During the summer, daily temperatures range from 15ºC to 45ºC, falling to freezing at night in the winter months. In 2001, the census recorded 275 people in Jigalong and 188 in Mugarinya.9
We collected morbidity data by examining medical records for children and adolescents (< 17 years) held at the local clinics. After the swimming pools opened in September 2000, we calculated annual morbidity rates from episodes occurring between 1 October and 30 September. All prescriptions for systemic antibiotics were recorded, and all episodes of ear, skin and respiratory tract infections (listed in Box 2) were documented. For comparison, we also documented changes in the rates of clinic visits for trauma, an illness category that we would not expect to decline after installation of a pool. We have previously reported a low incidence of pool-related trauma.6 Multiple attendances for treatment of an illness were counted as a single episode. However, if antibiotics were changed during the course of a single episode of illness, they were recorded as separate courses of antibiotics.
Two different methods of data selection were used:
The community-based selection method: This was used at Jigalong, where there was an ongoing study that has been described in detail.6 Our research team visited Jigalong every 6 months between September 2000 and February 2005 and examined the children who were present. For the current analysis, we used Jigalong clinic data for an individual child only if that child had been seen by the research team during one of its routine 6-monthly visits during the 12-month period, October to September. If the child had not been seen by the research team during one of the 12-month periods, clinic-based morbidity data for that year were recorded as “missing”. We considered the community-based selection method the most reliable assessment of which children were present during a 12-month period.
Written permission to access medical records had been obtained from parents or guardians in Jigalong when they gave consent for their children to participate in the regular surveys.6 Approval for investigators to access the medical files of children in Mugarinya was given by the local community council and the local public health authority. Ethical approval to conduct the study was given by the Western Australian Aboriginal Health Information and Ethics Committee and the Princess Margaret Hospital Ethics Committee (Perth, WA). The Confidentiality of Health Information Committee of the Health Department of WA approved access to clinic data.
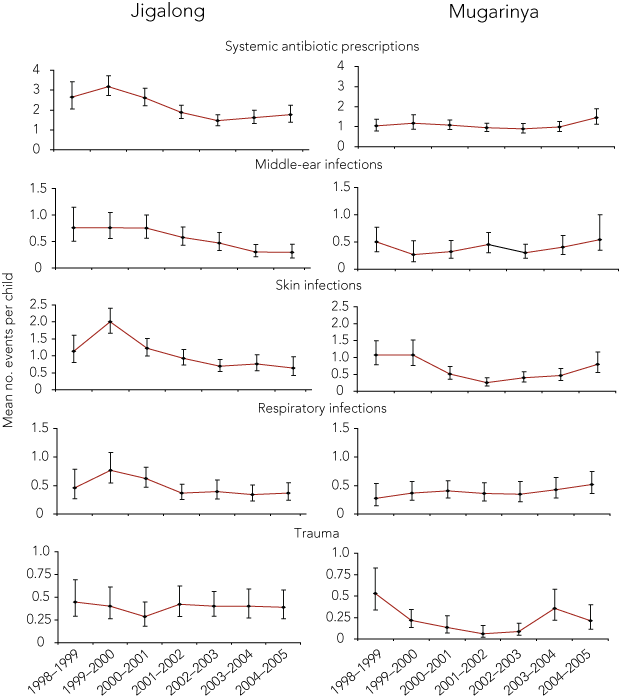
The mean number of middle-ear and respiratory infections per child at the start of the study was lower in Mugarinya than in Jigalong, but skin infections and trauma attendances were similar (Box 3).
In Jigalong, the rates of infectious diseases (adjusted for age and sex) declined significantly from the pre-pool year to 2004–2005 (Box 3, Box 4). With the clinic-based method, middle-ear infections decreased by 61%, skin infections by 68%, and respiratory infections by 52%. The community-based selection method gave similar reductions in morbidity. Antibiotic prescription rates decreased significantly 2 years after the pool was opened and remained low there-after. The prescription rate decreased by 45% from the pre-pool year to 2004–2005.
Log-linear trend analyses (Box 5) confirmed a significant decline in antibiotic prescriptions and infections in Jigalong from 1998 to 2005, irrespective of the selection method. In Mugarinya, trend analysis showed a decline in all skin infections, but no significant changes in rates of other infections, trauma-related events or antibiotic prescriptions.
There were 16 reports in Jigalong and 12 in Mugarinya of otitis externa (“swimmers ear”), a recognised problem among regular users of swimming pools,10,11 with no increase in prevalence over the course of the study.
Clinic attendances for skin infections in both Jigalong and Mugarinya declined after the installation of swimming pools. There was also a significant decline in attendance for middle-ear and respiratory infections in Jigalong, where attendance rates for these illnesses were higher in the pre-pool period than in Mugarinya. The reduction in middle-ear and skin infections in Jigalong confirms and extends our earlier findings over a 7-year period.6
However, in July 2001 pneumococcal conjugate vaccine was introduced in WA for children aged < 5 years, and by 2002 there was > 90% uptake in Jigalong and Mugarinya (Sally Connelly, Regional Immunisation Coordinator, Pilbara Population Health, personal communication, 2007). The vaccine program may have contributed to a reduction in pneumococcus-related respiratory illness, but is unlikely to have influenced attendance rates for middle-ear infections, because studies in the Northern Territory have not detected any effect of pneumococcal conjugate vaccine on the burden of severe middle-ear infections in Aboriginal communities.12 In our study, the reduction in possibly pneumococcus-related infections and other infections (eg, skin infections) actually began before a vaccine could have significantly affected morbidity rates. Furthermore, the reduction in disease burden was not equivalent in the two communities.
In view of high rates of infectious and chronic diseases, swimming pools in remote Aboriginal communities can have short-term and long-term advantages. Fewer prescriptions for antibiotics will reduce costs and antibiotic resistance, both of which are of concern in Aboriginal communities.13 Pyoderma is generally the result of group A streptococcal infections, and has been associated with rheumatic heart disease and glomerulonephritis.5,14-16 Aboriginal Australians have the highest documented rates of these illnesses worldwide.17,18 By reducing pyoderma, swimming pools may lower the incidence of the streptococcus-related chronic illnesses and the associated costs of renal dialysis and heart valve replacements.
Population mobility between Aboriginal communities is high and this can affect the interpretation of study results.19 Nevertheless, in Jigalong, community-based or clinic-based selection methods produced similar results. Therefore, we propose that evaluation of public health interventions for infectious diseases in children is possible using the clinic-based method in communities with resident staff and enough staff to ensure well kept clinic records.
2 Diagnoses included in infection categories,* recorded between 1998 and 2005 in Jigalong and Mugarinya
* Excluded were otitis externa, earache, foreign body, head lice, laceration, cough and asthma.
3 Mean rates, per child, of antibiotic prescriptions, infections and trauma, adjusted for age and sex, in two remote Western Australian Aboriginal communities, 1998–2005*
 |
4 Morbidity rate ratios (95% CIs), adjusted for age and sex, 2000–2005 relative to pre-pool year (1999–2000) in two remote Western Australian Aboriginal communities
- Desiree T Silva1
- Deborah Lehmann1
- Mary T Tennant1
- Peter Jacoby1
- Helen Wright2
- Fiona J Stanley1
- 1 Telethon Institute for Child Health Research, Centre for Child Health Research, University of Western Australia, Perth, WA.
- 2 Rural Clinical School of Western Australia, University of Western Australia, Kalgoorlie, WA.
We thank Helen Wright and Jenny Smith for assistance with data extraction from the notes and data entry, and Kirsten Alpers for editorial assistance. We thank the nursing and medical staff at Jigalong and Mugarinya and Kylie Carville for assisting us in obtaining the clinic records. This study was funded by the Western Australian Department of Housing and Works and the Western Australian Health Promotion Foundation (Healthway). Peter Jacoby and Deborah Lehmann are funded through National Health and Medical Council program grant 353514.
None identified.
- 1. Anderson I, Crengle S, Kamaka ML, et al. Indigenous health in Australia, New Zealand, and the Pacific. Lancet 2006; 367: 1775-1785.
- 2. Carville K, Lehmann D, Hall G, et al. Infection is the major component of the disease burden in Aboriginal and non-Aboriginal Australian children. Pediatr Infect Dis J 2007; 26: 210-216.
- 3. Freemantle CJ, Read AW, de Klerk NH, et al. Patterns, trends, and increasing disparities in mortality for Aboriginal and non-Aboriginal infants born in Western Australia, 1980–2001: population database study. Lancet 2006; 367: 1758-1766.
- 4. Reid J, Trompf P, editors. The health of Aboriginal Australia. Sydney: Harcourt Brace Jovanovich, 1991.
- 5. Currie BJ, Carapetis JR. Skin infections and infestations in Aboriginal communities in northern Australia. Australas J Dermatol 2000; 41: 139-145.
- 6. Lehmann D, Tennant MT, Silva DT, et al. Benefits of swimming pools in two remote Aboriginal communities in Western Australia: intervention study. BMJ 2003; 327: 415-419.
- 7. Morris PS, Leach AJ, Silberberg P, et al. Otitis media in young Aboriginal children from remote communities in Northern and Central Australia: a cross-sectional survey. BMC Pediatr 2005; 5: 27.
- 8. Turnidge JD, Bell JM, Collignon PJ. Rapidly emerging antimicrobial resistances in Streptococcus pneumoniae in Australia. Pneumococcal Study Group. Med J Aust 1999; 170: 152-155.
- 9. Trewin D. Population distribution, Aboriginal and Torres Strait Islander Australians, 2001. Canberra: Australian Bureau of Statistics, 2002. (ABS Cat. No. 4705.0.) http://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/85AB4A6668629B77CA256BE400026A25/$File/47050_2001.pdf (accessed Dec 2007).
- 10. van Asperen IA, de Rover CM, Schijven JF, et al. Risk of otitis externa after swimming in recreational fresh water lakes containing Pseudomonas aeruginosa. BMJ 1995; 311: 1407-1410.
- 11. van Balen FA, Smit WM, Zuithoff NP, Verheij TJ. Clinical efficacy of three common treatments in acute otitis externa in primary care: randomised controlled trial. BMJ 2003; 327: 1201-1205.
- 12. McKenzie GA, Carapetis JR, Leach AJ, et al. Pneumococcal vaccination and otitis media in Australian Aboriginal infants [abstract PO 1210]. 5th International Symposium on Pneumococci and Pneumococcal Diseases; 2006 Apr 2–6; Alice Springs.
- 13. Grimwood K, Collignon PJ, Currie BJ, et al. Antibiotic management of pneumococcal infections in an era of increased resistance. J Paediatr Child Health 1997; 33: 287-295.
- 14. Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet 2005; 366: 155-168.
- 15. Steer AC, Danchin MH, Carapetis JR. Group A streptococcal infections in children. J Paediatr Child Health 2007; 43: 203-213.
- 16. Streeton CL, Hanna JN, Messer RD, Merianos A. An epidemic of acute post-streptococcal glomerulonephritis among aboriginal children. J Paediatr Child Health 1995; 31: 245-248.
- 17. Carapetis JR, Currie BJ, Kaplan EL. Epidemiology and prevention of group A streptococcal infections: acute respiratory tract infections, skin infections, and their sequelae at the close of the twentieth century. Clin Infect Dis 1999; 28: 205-210.
- 18. Carapetis JR, Currie BJ. Clinical epidemiology of rheumatic fever and rheumatic heart disease in tropical Australia. Adv Exp Med Biol 1997; 418: 233-236.
- 19. Taylor J. Short-term Indigenous population mobility and service delivery. CAEPR discussion paper 118. Canberra: Centre for Aboriginal Economic Policy Research, Australian National University, 1996.





Abstract
Objective: To determine whether installation of swimming pools in remote Aboriginal communities reduces infection-related outpatient attendances and prescription of antibiotics.
Design and setting: Swimming pools were opened in Jigalong and Mugarinya, Western Australia, in September 2000. We examined local clinic records to document illnesses occurring in children and adolescents under 17 years of age between 1998 and 2005. In Jigalong, we examined records of those enrolled in an ongoing study evaluating the effect of swimming pools on health. In Mugarinya, we examined clinic records of those residing there permanently.
Main outcome measures: Clinic attendance rates for skin, middle-ear and respiratory tract infections and trauma, and prescription rates for antibiotics were analysed by using a community-based selection method in Jigalong, and a clinic-based selection method in both communities for comparison of the two communities and the two methods.
Results: We examined records of 131 children in Jigalong and 128 children in Mugarinya. After the pools had been installed, clinic attendance rates for skin infections declined by 68% in Jigalong and by up to 77% in Mugarinya. In Jigalong (where the pre-pool prevalence of infections was higher than in Mugarinya), rates of antibiotic prescription declined by 45%, as did clinic attendance for middle-ear infections (61% reduction) and respiratory tract infections (52% reduction).
Conclusion: Swimming pools in remote communities are associated with reduced prevalence of skin infections. Where disease prevalence is high, pools are also associated with reduced rates of antibiotic prescriptions and middle-ear and respiratory tract infections. In communities with resident health staff, examination of clinic records is an efficient method of monitoring the effects of public health interventions on the burden of infectious diseases.