The most common malignant tumours of the oesophagus are primary oesophageal tumours.1 Of these, over 90% are squamous cell carcinomas (SCCs) and adenocarcinomas (ACs) of the oesophagus and gastro-oesophageal junction. Although SCC is the most prevalent type of oesophageal cancer throughout the world, its incidence has stabilised over the past 20–30 years. By contrast, the incidence of AC of the oesophagus and gastro-oesophageal junction has dramatically increased in the United States, Europe and Australia, particularly in men.2-7 Unfortunately, symptoms often manifest late in the course of the disease, leading to diagnosis at a fairly advanced stage and thus a poor prognosis.
An association between alcohol and tobacco intake and SCC and AC is well established, although the mechanism is unclear.3,8-10 Barrett’s oesophagus, which is a consequence of chronic gastro-oesophageal reflux disease (GORD), is an important risk factor for AC of the oesophagus.3,8,10,11 GORD is also an independent risk factor for AC of the oesophagus.12
The NSW CCR is run according to the rules of the International Association of Cancer Registries,13 and is the only Australian cancer registry to record degree of spread at first diagnosis for all solid malignant tumours.14 For each case, the degree of spread is assigned by the NSW CCR to one of four stages: localised, regional, distant (metastatic) or unknown. Degree of spread is defined as the maximum extent of disease, based on all diagnostic and therapeutic evidence received within 4 months of diagnosis. It follows the international coding guidelines for summary stage adopted by several international groups, including the World Health Organization and the International Association of Cancer Registries.13
Cases in the sample were assigned to geographical remoteness categories using the Accessibility/Remoteness Index of Australia (ARIA+).15 Each case was first allocated to a local government area (LGA) based on the patient’s residential address at the time of diagnosis. ARIA+ categories were then applied via LGA classifications. LGAs were defined according to 2001 census information from the Australian Bureau of Statistics (ABS).15
Socioeconomic status of patients was estimated using the Index of Relative Socio-Economic Disadvantage (IRSD), one of four Socio-Economic Indexes for Areas created by the ABS.16 The IRSD score for each LGA was taken from 2001 census information and categorised into population-weighted quintiles.
Age-standardised rates of cancer incidence and survival were calculated using the 2001 Australian population as the standard. Population estimates for NSW as a whole, as well as the standard Australian population and LGA populations, were obtained from the ABS (estimated residential population) and accessed via the Health Outcomes Indicator Statistical Toolbox, a population data access and analysis facility available through NSW Health. Trend analyses were performed using the Joinpoint Regression Program provided by the US National Cancer Institute (http://srab.cancer.gov/joinpoint).
Factors associated with diagnosis of AC were examined using logistic regression and reported as crude (univariate) and adjusted odds ratios (ORs), with statistical significance reported at the P < 0.5 (two-tailed) level.
All statistical analysis was performed using SAS software, version 9 (SAS Institute Inc, Cary, NC).
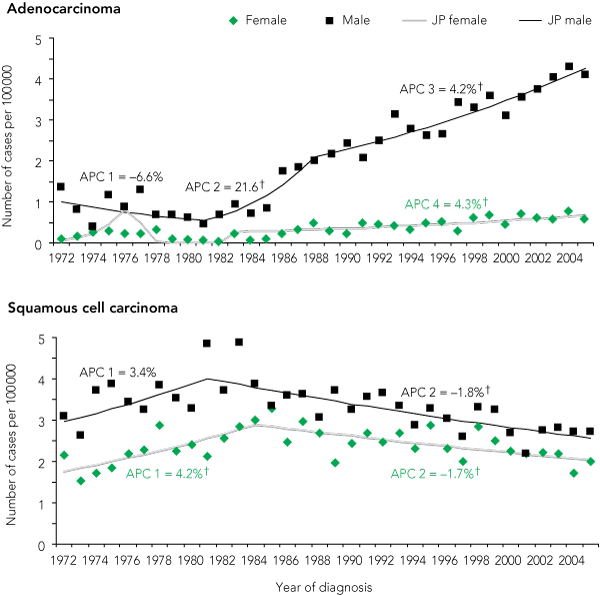
Over the period 1972–2005, 2445 cases of AC and 6518 cases of SCC and other oesophageal cancers were recorded in the NSW CCR (Box 1). Age-standardised incidence rates of AC increased significantly for both males and females (Box 2). In males, the annual percentage change in AC age-standardised incidence between 1972 and 1981 (estimated) was not significant (– 6.6% [95% CL, – 13.4%, 0.8%]); between 1981 and 1988 (estimated), there was a sharp increase in AC incidence (annual percentage change, 21.6% [95% CL, 10.8%, 33.5%]; P < 0.001); and from 1988 (estimated) AC incidence increased significantly, though at a lower rate (annual percentage change, 4.2% [95% CL, 2.7%, 5.8%]; P < 0.001). In females, the age-standardised incidence of AC increased significantly from 1983 (estimated), with an annual percentage change of 4.3% (95% CL, 1.8%, 7.0%) between 1983 and 2005.
A diagnosis of AC rather than SCC or other cancers was associated with being male (adjusted OR [AOR], 4.37 [95% CL, 3.84, 4.98]; P < 0.001); having a younger age at diagnosis (P trend < 0.001); progressive year of diagnosis (P trend < 0.001); having higher socioeconomic status (P trend < 0.001); living in an inner regional area (AOR, 1.26 [95% CL, 1.11, 1.43]; P < 0.001) or an outer regional area (AOR, 1.19 [95% CL, 1.00, 1.41]; P = 0.05) compared with a major city; and having regional disease spread (AOR, 1.60 [95% CL, 1.38, 1.85]; P < 0.001) or distant (metastatic) disease spread (AOR, 2.12 [95% CL, 1.82, 2.48]; P < 0.001) rather than localised disease (Box 1).
One-year absolute survival proportions for patients diagnosed with AC between 1972 and 2004 were 48.5% (95% CL, 44.5%, 52.4%) for localised cancer; 42.6% (95% CL, 38.6%, 46.6%) for cancer with regional spread; and 12.0% (95% CL, 9.3%, 15.1%) for cancer with distant spread (Box 3).
Proportional regression analysis (Box 4) showed that the adjusted hazard ratio (AHR) for poor all-cause survival was three times higher in AC patients with distant cancer spread than in patients with localised disease (AHR, 3.10 [95% CL, 2.70, 3.56]; P < 0.001). Increasing age at diagnosis (P < 0.001), earlier period of diagnosis (P < 0.001), and living in a regional area rather than a major city (P trend = 0.09) were also associated with poorer survival. There was no association between sex or socioeconomic status and absolute survival in patients with AC.
The incidence of oesophageal AC has increased in NSW since 1972, in line with trends in Australia as a whole7 and throughout the Western world.3-6 A falling prevalence of Helicobacter pylori infection may contribute to the increasing incidence of GORD, and hence AC. Another possible factor contributing to the increase in AC is the rise in obesity/body mass index with age that has occurred in recent times.8,9,17,18 It has been suggested that increased abdominal pressure due to obesity can cause GORD. Furthermore, the effect of obesity and reflux has been shown to be synergistic — that is, the mechanism of association between obesity and AC is not necessarily only via GORD.8
Rates of obesity and excessive alcohol consumption have been reported to be higher in rural than metropolitan areas.18-20 These may be contributing factors to the higher odds of being diagnosed with oesophageal AC in regional areas. Lack of access to specialists in regional areas may also correlate with the increased incidence of AC in rural communities. Programs of “open-access” endoscopy, allowing general practitioners to refer patients directly for diagnostic endoscopy (in accordance with American Society for Gastrointestinal Endoscopy guidelines) without an initial gastrointestinal examination, have been advocated to reduce costs and waiting times for remote and rural patients.21
The higher incidence of oesophageal AC in males may reflect the fact that men are less likely than women to consult a GP when they experience early warning signs of AC (such as dysphagia and weight loss).22
Some authors have recommended surveillance of patients with Barrett’s oesophagus who are suitable for undergoing oesophagectomy, as it is cost-effective with regard to long-term outcome, with the interval between endoscopy procedures dependent on the presence or absence of dysplasia.23 However, other studies of surveillance of Barrett’s oesophagus have produced mixed results.24-26 Endoscopic surveillance can monitor the progression of metaplasia to low-grade dysplasia, high-grade dysplasia or AC.27 The cost-effectiveness of undergoing such a process in NSW needs to be evaluated.
Our study had a number of limitations. As cancer data were not linked to information on behavioural risk factors, we could only speculate that the increase in oesophageal cancer, particularly AC, was associated with increases in body mass index and obesity in the NSW population.18,28 Similarly, without linkage to disease registers or other data sources, it could only be speculated that the increase in AC was related to an increase in GORD. However, cohort studies overseas and in Australia have demonstrated a link between behavioural and environmental risk factors and oesophageal AC.8,9
A further limitation of our study was that ARIA+ and IRSD scores were assigned to LGA boundaries defined according to 2001 census information. Although year of diagnosis was included in the modelling to account for changes over time, boundary changes could not be accounted for, as scores would be calculated based on populations that differed with respect to accessibility and socioeconomic status. Thus there was the potential for misclassification bias, particularly in areas that were considered “regional” 20–30 years ago but are now part of a “major city”. However, although other geographical classifications have been used by the ABS in the past, remoteness classification based on standard geographical classification did not exist before 2001.29 Furthermore, as a historical database, it is important that consistent numerator and denominator populations be applied to the NSW CCR. Reporting based on 2001 geographic boundaries is consistent with using the 2001 Australian population as the standard population.
Finally, the NSW CCR is yet to be evaluated for validity and completeness, but this will be done in the near future. Nevertheless, two measures of data quality are already available: the proportion of cases with histological verification and the proportion with a death certificate only.2 The proportion of cases with histological verification in the NSW CCR has improved from 69% in 1972 to 87% in 2005, and has consistently been over 80% since 1980. Such percentages are only slightly lower than those of cancer registries in Denmark and the US.2 Similarly, the proportion of cases with a death certificate only has been comparable to that of other registries at < 2% since 1972 (with the exception of the period 1983–1990, when active follow-up of cases was reduced due to lack of resources).
1 Factors associated with a diagnosis of adenocarcinoma and other cancers of the oesophagus in New South Wales residents, 1972–2005
2 Age-standardised incidence and joinpoint analysis of oesophageal adenocarcinoma and squamous cell carcinoma in New South Wales residents, by sex, 1972–2005*
 | |||||||||||||||
3 Kaplan–Meier curve:* all-cause survival in New South Wales residents with oesophageal adenocarcinoma, by degree of cancer spread at diagnosis
 | |||||||||||||||
Received 12 October 2008, accepted 3 May 2009
- Efty P Stavrou1
- Heather J McElroy1
- Deborah F Baker1
- Garett Smith2
- James F Bishop1
- 1 Cancer Institute NSW, Sydney, NSW.
- 2 Royal North Shore Hospital, Sydney, NSW.
None identified.
- 1. Ginsberg GG, Fleisher DE. Tumors of the esophagus. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s gastrointestinal and liver disease: pathophysiology, diagnosis, management. 8th ed. Philadelphia: Saunders Elsevier, 2006.
- 2. Tracey EA, Baker D, Chen W, et al. Cancer in New South Wales: incidence, mortality and prevalence report 2005. Sydney: Cancer Institute NSW, 2007.
- 3. Chou JC, Gress FG. Esophageal tumors. In: Friedman SL, McQuaid K, Grendell JH, editors. Current diagnosis and treatment in gastroenterology. 2nd ed. New York: McGraw-Hill, 2003.
- 4. Pera M, Cameron AJ, Trastek VF, et al. Increasing incidence of adenocarcinoma of the esophagus and esophago-gastric junction. Gastroenterology 1993; 104: 510-517.
- 5. El-Serag HB, Sonnenberg A. Opposing time trends of peptic ulcer disease and reflux disease. Gut 1998; 43: 327-331.
- 6. Crane SJ, Locke GR 3rd, Harmsen WS, et al. The changing incidence of oesophageal and gastric adenocarcinoma by anatomic sub-site. Aliment Pharmacol Ther 2007; 25: 447-453.
- 7. Lord RV, Law MG, Ward RL, et al. Rising incidence of oesophageal adenocarcinoma in men in Australia. J Gastroenterol Hepatol 1998; 13: 356-362.
- 8. Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut 2008; 57: 173-180.
- 9. De Jonge PJ, Wolters LM, Steyerberg EW, et al. Environmental risk factors in the development of adenocarcinoma of the oesophagus or gastric cardia: a cross-sectional study in a Dutch cohort. Aliment Pharmacol Ther 2007; 26: 31-39.
- 10. Spechler SJ. Esophageal diseases. In: Friedman SL, McQuaid K, Grendell JH, editors. Current diagnosis and treatment in gastroenterology. 2nd ed. New York: McGraw-Hill, 2003.
- 11. Posner MC, Forastiere AA, Minsky BD. Cancers of the gastrointestinal tract. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. 7th ed. Philadelphia: Lippincott, Williams and Wilkins, 2005.
- 12. Crane SJ, Locke GR 3rd, Harmsen WS, et al. Subsite-specific risk factors for esophageal and gastric adenocarcinoma. Am J Gastroenterol 2007; 102: 1596-1602.
- 13. Esteban D, Whelan S, Laudico A, Parkin D, editors. Manual for cancer registry personnel. Lyon: International Agency for Research on Cancer, 1995. (IARC Technical Report No 10.)
- 14. Barraclough H, Morrell S, Arcorace M, et al. Degree-of-spread artefact in the New South Wales Central Cancer Registry. Aust N Z J Public Health 2008; 32: 414-416.
- 15. GISCA. About ARIA+ (Accessibility/Remoteness Index of Australia). http://www.gisca.adelaide.edu.au/products_services/ariav2_about.html (accessed Jun 2009).
- 16. Pink B. An Introduction to Socio-Economic Indexes for Areas (SEIFA). Information paper. Canberra: Australian Bureau of Statistics, 2006. (ABS Cat. No. 2039.0.)
- 17. Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007; 335: 1134-1144.
- 18. Population Health Division. The health of the people of New South Wales. Report of the Chief Health Officer, 2006. Sydney: NSW Department of Health, 2006.
- 19. Janus ED, Laatikainen T, Dunbar JA, et al. Overweight, obesity and metabolic syndrome in rural southeastern Australia. Med J Aust 2007; 187: 147-152. <MJA full text>
- 20. Di Sipio T, Rogers C, Newman B, et al. The Queensland Cancer Risk Study: behavioural risk factor results. Aust N Z J Public Health 2006; 30: 375-382.
- 21. Hughes-Anderson W, Rankin SL, House J, et al. Open access endoscopy in rural and remote Western Australia: does it work? ANZ J Surg 2002; 72: 699-703.
- 22. Harris MF, McKenzie S. Men’s health: what’s a GP to do? Med J Aust 2006; 185: 440-444. <MJA full text>
- 23. Aldulaimi DM, Cox M, Nwokolo CU, et al. Barrett’s surveillance is worthwhile and detects curable cancers. A prospective cohort study addressing cancer incidence, treatment outcome and survival. Eur J Gastroenterol Hepatol 2005; 17: 943-950.
- 24. Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost–utility analysis. Ann Intern Med 2003; 138: 176-186.
- 25. Mashimo H, Wagh MS, Goyal RK. Surveillance and screening for Barrett oesophagus and adenocarcinoma. J Clin Gastroenterol 2005; 39 (4 Suppl 2): S33-S41.
- 26. Macdonald CE, Wicks AC, Playford RJ. Final results from 10 year cohort of patients undergoing surveillance for Barrett’s oesophagus: observational study. BMJ 2000; 321: 1252-1255.
- 27. Ajani J, Bekaii-Saab T, D’Amico TA, et al. NCCN clinical practice guidelines in oncology. Esophageal cancer. Fort Washington, Pa: National Comprehensive Cancer Network, 2008. http://www.nccn.org/professionals/physician_gls/PDF/esophageal.pdf (accessed Jul 2009).
- 28. Population Health Division. The health of the people of New South Wales. Report of the Chief Health Officer, 2008: summary report. Sydney: NSW Department of Health, 2008.
- 29. Australian Bureau of Statistics. ASGC remoteness classification: purpose and use. Canberra: ABS, 2003. (Census Paper No. 03/01.)





Abstract
Objective: To investigate trends in the incidence of adenocarcinoma (AC) of the oesophagus in New South Wales, factors associated with a diagnosis of AC, and factors associated with survival of patients with AC.
Design and setting: We examined all cases of invasive oesophageal cancer recorded in the NSW Central Cancer Registry from 1972 to 2005. The Accessibility/Remoteness Index of Australia was used to assess geographical remoteness and the Index of Relative Socio-Economic Disadvantage to assess socioeconomic status.
Main outcome measures: Incidence of AC; factors associated with diagnosis of AC and survival of patients with AC.
Results: The overall incidence of oesophageal AC in NSW increased in both males and females (annual percentage change, 4.2% [95% CL, 2.7%, 5.8%] in males [1988–2005] and 4.3% [95% CL, 1.8%, 7.0%] in females [1983–2005]). A diagnosis of AC was significantly associated with being male (adjusted odds ratio [AOR], 4.37 [95% CL, 3.84, 4.98]; P < 0.001); a younger age at diagnosis (P trend < 0.001); having distant rather than localised disease spread (AOR, 2.12 [95% CL, 1.82, 2.48]; P < 0.001); higher socioeconomic status (P trend < 0.001); and living in an inner regional area (AOR, 1.26 [95% CL, 1.11, 1.43]; P < 0.001) or outer regional area (AOR, 1.19 [95% CL, 1.00, 1.41]; P = 0.05) compared with a major city. Early diagnosis of AC was associated with substantial improvement in survival outcomes: patients with metastatic disease at diagnosis had a three times greater risk of dying than those with localised AC at diagnosis.
Conclusion: The incidence of AC is increasing in NSW. Possible contributing factors include increasing obesity, which is associated with increased incidence of gastro-oesophageal reflux disease. Survival may be improved by diagnosis at an earlier stage and changes in modifiable risk factors (eg, smoking, diet, exercise).