A widely discussed deterrent to genetic testing is the concern that test results might adversely affect one’s prospect of purchasing life, trauma, or disability insurance policies issued by life insurance companies or sickness and accident insurance policies issued by general insurance companies (see Box 1).1-4 In Australia, while genetic information has no effect on health insurance or group insurance (available with superannuation plans), applicants for life, trauma, disability or sickness and accident insurance have a statutory duty under the Insurance Contracts Act 1984 (Cwlth) to disclose known and relevant information, including genetic test results and family history of disease. Provided an insurer follows sound actuarial practices and does not violate anti-discrimination laws, it may refuse applicants coverage or charge higher premiums based on the information disclosed.5,6 Instances of genetic discrimination have recently been reported in Australia.7
Little is known about how concerns regarding insurance implications influence people’s willingness to undergo genetic testing. In a study of 300 Jewish Australians, 94% indicated they would have testing for the APC I1307K variant (indicating a small increased risk of colorectal cancer), but 54% were concerned about the possibility of discrimination by insurance companies as a result of doing so.8 In the wider Australian community and for other genetic syndromes, the extent of these concerns has not been measured. Insurance-related apprehension about genetic testing could have troubling public health consequences, particularly when awareness of genetic status could trigger potentially life-saving lifestyle changes or clinical interventions.
For over 10 years, it has been possible to test people to determine if they carry a germline mutation in the mismatch repair (MMR) genes MLH1, MSH2, MSH6 and PMS2.9,10 About one in every 3000 people of Anglo-Saxon descent carry a germline MMR gene mutation11 and are at greatly increased risk of colorectal cancer (especially at a young age) and some other cancers.12,13 Current recommendations for the clinical care of mutation carriers include annual or biennial colonoscopy and subtotal colectomy for those undergoing surgical resection for colon cancer.14 Screening of the rectum and large bowel of unaffected carriers has been shown to reduce the risk of colorectal cancer by 56%.15 Screening and early identification of carriers is therefore a potentially highly cost-effective way to reduce the burden of colorectal cancer.
The Victorian Colorectal Cancer Family Study (VCCFS) offered an opportunity to assess the influence of providing information on insurance implications on the uptake of genetic testing for MMR mutations. Partway through the VCCFS, we became aware that legal duties of disclosure to insurance companies extended to people who learned about their genetic status in the course of such studies. The study protocol was therefore changed to inform participants of the insurance implications, whereas participants recruited earlier had not been given this information. Using this as a natural experiment, we analysed whether the uptake of genetic testing by participants changed after the protocol amendment.
The VCCFS is a population-based case–control family study for which recruitment was conducted between 1993 and 1997. It involves 131 adults diagnosed with colorectal cancer before the age of 45 years (case-probands) and their first- and second-degree relatives. A total of 18 case-probands were found to carry a germline mutation in an MMR gene.12,16
As required by the human research ethics committees of the University of Melbourne and the Cancer Council Victoria, all case-probands found to carry a mutation, and all their relatives who had participated in the VCCFS, were given the opportunity to learn of their mutation status. They were told that they could attend a family cancer clinic (FCC), at no cost to themselves, to receive genetic counselling and, if they wished, obtain genetic testing by an accredited testing laboratory. All contact with participants and FCCs by the VCCFS was recorded to enable tracking of participants’ and clinics’ follow-up activity, including any clinic attendances and receipt of test results.
The principal outcome was whether or not participants obtained genetic testing results through a genetic counsellor and accredited clinical service, and the principal predictor was whether the decision to obtain genetic test results was made under the original or modified version of the protocol. Using data available through the VCCFS, we also examined other explanatory variables because of their potential to influence genetic testing behaviour: participants’ sex, age and education; whether they had a previous colonoscopy; and whether they had a personal history of colorectal cancer or a family history (two or more first-degree relatives with any cancer versus one or no first-degree relatives with any cancer).17,18 Participants who were lost to follow-up, had obtained genetic test results before enrolment in the VCCFS, or had died and not previously obtained genetic test results were excluded from the analysis.
Proportions were compared using Pearson χ2 test statistics, or Fisher’s exact test when appropriate. Continuous measures were compared using the t test. Relationships between the binary outcome and potential explanatory variables were analysed using logistic regression. A parsimonious model with no interactions was determined by forwards selection and confirmed by backwards elimination. Two-way interaction terms were then tested.
As participants recruited using the original protocol had more time to obtain genetic test results, we also conducted a sensitivity analysis by restricting the analysis to participants who received their genetic test results within 4 years of the year in which their version of the protocol commenced.
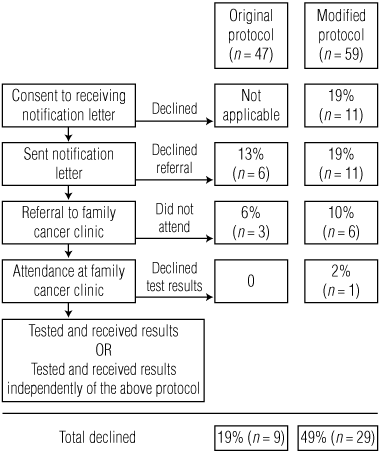
Under the original protocol, we attempted to send notification letters to 49 people from 11 mutation-carrying families; one was deceased and one could not be contacted. Nine of the remaining 47 did not obtain genetic test results, either because they declined a referral to an FCC (6) or were referred but did not attend (3) (Box 2). Of the 38 participants who obtained their results, 13 did so independently of the VCCFS and 25 did so via a VCCFS referral to an FCC.
Under the modified protocol, we attempted to send notification letters to 66 people from 14 mutation-carrying families; three were deceased and four could not be contacted. Twenty-nine of the remaining 59 declined to obtain genetic test results (Box 2). Opt-outs occurred at each stage: 11 participants chose not to receive a notification letter, a further 11 declined the referral to an FCC, six sought a referral to an FCC but did not attend, and one attended but chose not to obtain the test results. Of the 30 participants who obtained test results, five did so independently of the VCCFS.
There were no differences between participants under the two versions of the protocol with respect to sex, age, family history of colorectal cancer or education level (Box 3), but participants under the original protocol were more likely to have had a previous colonoscopy (77% v 58%; P = 0.04) or colorectal cancer (43% v 24%; P = 0.04).
Participants under the modified protocol were 2.6 times more likely to decline their genetic test results (49% v 19%; P = 0.002). This association remained after adjusting for the other measured factors (odds ratio [OR], 4.06; 95% CI, 1.32–12.45) (Box 4). In addition, the odds of declining genetic test results increased by 6% with each year of age (OR, 1.06; 95% CI, 1.03–1.10), and participants with a prior colonoscopy were less likely to decline (OR, 0.25; 95% CI, 0.09–0.70). No other factor that was examined and no two-way interactions among those factors were associated with the decision to obtain genetic test results (Box 4).
When the analysis was restricted to participants who received notification within 4 years of protocol commencement, only four participants were excluded and the results were only slightly altered; the OR for declining to obtain genetic test results under the modified protocol became 3.34 (95% CI, 1.13–9.85).
This population-based study showed that the uptake of genetic testing by research participants at potentially heightened risk of colorectal cancer decreased dramatically after the notification process was modified to include information about the potentially negative implications of receiving genetic information on new life, trauma or disability insurance policies and new or renewable sickness and accident insurance policies. Multivariate analyses showed that the odds that participants would opt out along the pathway to receiving genetic test results were four times higher when they were provided with this information. Participants under the modified protocol had an extra opportunity at the start of the process to decline genetic information, and 19% did so. The proportion of participants who declined at all other steps was larger under the modified protocol than the original protocol. Although we cannot claim that the reduction in uptake was caused by changes to the procedure of informing participants, the results are consistent with such a relationship.
One potential limitation of this study was its small sample size; nevertheless, the associations identified were highly statistically significant. Although the study was conducted principally in one state, family members could have lived anywhere in Australia or abroad. The homogeneity of the Australian population with respect to genetic testing and treatment suggests the results would apply to the wider community.
The population-based design of the study was a major strength, avoiding potential biases associated with using members of self-selected families in which some members might be especially motivated to learn of their genetic risk status. Consequently, most participants offered genetic information in this research (64%–70%) had a weak or no family history of colorectal cancer. All those referred to an FCC had a mutation detected in their family, making them an important sample within which to study genetic test-seeking behaviour, because they represent people who can change their behaviour to improve their medical care.
The 81% uptake of genetic testing under our original protocol is similar to that reported in Finland,19 where concerns about insurance implications appear to be minimal. The 51% uptake under our modified protocol is closer to the 43%–58% reported for first-degree relatives of colon cancer patients in the United States,17,20,21 where insurance issues loom larger.
In the United Kingdom, the insurance industry has voluntarily undertaken to allow customers not to disclose adverse results of predictive genetic testing for most life, critical illness and income protection insurance policies.22 The industry has also declared a moratorium on asking customers “to disclose any predictive or diagnostic genetic test results acquired as part of clinical research”. In the US, the Genetic Information Nondiscrimination Act was passed in 2008, prohibiting health insurance companies from using genetic data to set premiums or determine enrolment eligibility.
Our findings, coupled with these developments abroad, raise important questions for the Australian insurance industry. If the industry’s position on genetic information23 deters individuals from obtaining test results, the clinical and public health consequences could be damaging. This is particularly true when awareness of one’s genetic status opens the way to action that can effectively reduce risks of adverse health conditions.
A 2003 review of the use and protection of human genetic information, conducted by the Australian Law Reform Commission and the Australian Health Ethics Committee, concluded that no special prohibitions on use of genetic information by the insurance industry were justified.2 Nonetheless, the Human Genetics Advisory Committee has been established to keep a “watching brief” over this area and provide ongoing advice to the Australian Government. We call on this Committee and the Australian insurance industry to reconsider the use of genetic information in circumstances where there is clear potential for the information to be used to reduce morbidity and mortality.
1 Insurance definitions
Life insurance: provides for the payment of an agreed lump sum in the event of death of the insured.
Disability or income protection insurance: provides for regular sums to be paid while an insured person is unable to work due to sickness or injury.
Trauma insurance: provides for the payment of an agreed lump sum if the insured person is diagnosed with one of a list of specified conditions.
The above three policies are “guaranteed renewable” so, once approved, one is not obliged to inform the insurance company of any change in circumstances.
Sickness and accident insurance: is similar to disability insurance but is offered by general insurance companies and is renewable. This means that any change in circumstances (such as new genetic information) must be declared at renewal.
3 Characteristics of participants under each protocol,* and statistical significance of test of no difference between protocols
Characteristic |
Original protocol (n = 47) |
Modified protocol (n = 59) |
P |
||||||||||||
Male |
25 (53%) |
24 (41%) |
0.24 |
||||||||||||
Age in years, mean (SD) |
46.8 (13.7) |
51.0 (13.9) |
0.13 |
||||||||||||
Previous colorectal cancer |
20 (43%) |
14 (24%) |
0.04 |
||||||||||||
Moderate–strong family history† |
15 (32%) |
22 (37%) |
0.68 |
||||||||||||
At least one previous colonoscopy |
36 (77%) |
34 (58%) |
0.04 |
||||||||||||
Higher education |
24 (51%) |
32 (54%) |
0.85 |
||||||||||||
* No. (%) of participants unless otherwise indicated. † ≥ 2 first-degree relatives with any cancer. |
|||||||||||||||
4 Association between potential explanatory variables and declining genetic test results
|
Genetic test results* |
Odds ratio (OR) for declining genetic test results |
|||||||||||||
Variable |
Declined (n = 38) |
Obtained (n = 68) |
Crude OR (95% CI) |
P |
Adjusted OR† (95% CI) |
P |
|||||||||
Original protocol (referent) |
9 (19%) |
38 (81%) |
1.00 |
|
1.00 |
|
|||||||||
Modified protocol |
29 (49%) |
30 (51%) |
4.08 (1.68–9.18) |
0.002 |
4.06 (1.32–12.45) |
0.014 |
|||||||||
Female (referent) |
21 (37%) |
36 (63%) |
1.00 |
|
1.00 |
|
|||||||||
Male |
17 (35%) |
32 (65%) |
1.10 (0.49–2.44) |
0.82 |
1.05 (0.38–2.85) |
0.93 |
|||||||||
Age in years, mean (SE) |
56.1 (14.2) |
45.2 (12.2) |
1.06‡ (1.03–1.10) |
< 0.001 |
1.06‡ (1.03–1.10) |
0.001 |
|||||||||
No colorectal cancer (referent) |
29 (40%) |
43 (60%) |
1.00 |
|
1.00 |
|
|||||||||
Colorectal cancer |
9 (26%) |
25 (74%) |
0.53 (0.22–1.31) |
0.17 |
0.59 (0.19–1.86) |
0.37 |
|||||||||
Weak–no family history§ (referent) |
27 (39%) |
42 (61%) |
1.00 |
|
1.00 |
|
|||||||||
Moderate–strong family history¶ |
11 (30%) |
26 (70%) |
0.66 (0.28–1.55) |
0.34 |
0.45 (0.16–1.29) |
0.14 |
|||||||||
No previous colonoscopy (referent) |
21 (58%) |
15 (42%) |
1.00 |
|
1.00 |
|
|||||||||
Previous colonoscopy |
17 (24%) |
53 (76%) |
0.23 (0.10–0.54) |
0.001 |
0.25 (0.09–0.70) |
0.008 |
|||||||||
High school or less (referent) |
22 (44%) |
28 (56%) |
1.00 |
|
1.00 |
|
|||||||||
Higher education |
16 (29%) |
40 (71%) |
0.51 (0.23–1.14) |
0.10 |
0.42 (0.15–1.20) |
0.11 |
|||||||||
* No. (%) of participants unless otherwise indicated. † Adjusted for all other variables. ‡ Per year of age above 20 years. § ≤ 1 first-degree relative with any cancer. ¶ ≥ 2 first-degree relatives with any cancer. |
|||||||||||||||
Received 21 December 2008, accepted 30 April 2009
- Louise A Keogh1
- Christine M van Vliet2
- David M Studdert3
- Judith A Maskiell4
- Finlay A Macrae5
- D James St John5
- Clara L Gaff6,7
- Mary Anne Young8
- Melissa C Southey9
- Graham G Giles10
- Doreen A Rosenthal1,4
- John L Hopper4
- Mark A Jenkins4
- 1 Key Centre for Women’s Health in Society, University of Melbourne, Melbourne, VIC.
- 2 Faculty of Medicine, University of New South Wales, Sydney, NSW.
- 3 Centre for Health Policy, Programs and Economics, University of Melbourne, Melbourne, VIC.
- 4 Centre for MEGA Epidemiology, University of Melbourne, Melbourne, VIC.
- 5 Department of Colorectal Medicine and Genetics, Royal Melbourne Hospital, Melbourne, VIC.
- 6 Genetic Health Services Victoria, Melbourne, VIC.
- 7 Faculty of Medicine, University of Melbourne, Melbourne, VIC.
- 8 Familial Cancer Centre, Peter MacCallum Cancer Institute, Melbourne, VIC.
- 9 Genetic Epidemiology Laboratory, University of Melbourne, Melbourne, VIC.
- 10 Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, VIC.
* Equal first authors.
We gratefully acknowledge Dr Letitia Smith for her work on this study; Professor Margaret Otlowski for providing useful comments; and the genetic counsellors for their help in guiding us through the clinical referral process. Louise Keogh was supported by a National Health and Medical Research Council (NHMRC) Australian Research Training Fellowship. David Studdert was supported by a Federation Fellowship from the Australian Research Council. Melissa Southey is an NHMRC Senior Research Fellow. John Hopper is an Australia Fellow of the NHMRC.
None identified.
- 1. Anderlik MR, Rothstein MA. Privacy and confidentiality of genetic information: what rules for the new science? Annu Rev Genomics Hum Genet 2001; 2: 401-433.
- 2. Australian Law Reform Commission, Australian Health Ethics Committee. Essentially yours: the protection of human genetic information in Australia (ALRC 96). Sydney: ALRC, 2003.
- 3. Taylor S, Treloar S, Barlow-Stewart K, et al. Investigating genetic discrimination in Australia: a large-scale survey of clinical genetics clients. Clin Genet 2008; 74: 20-30.
- 4. Lynch EL, Doherty RJ, Gaff CL, et al. “Cancer in the family” and genetic testing: implications for life insurance. Med J Aust 2003; 179: 480-483. <MJA full text>
- 5. Keays DA. The legal implications of genetic testing: insurance, employment and privacy. J Law Med 1999; 6: 357-372.
- 6. Otlowski MFA. Resolving the conundrum: should insurers be entitled to access to genetic test information? Insur Law J 2000; 11: 193-215.
- 7. Barlow-Stewart K, Taylor SD, Treloar SA, et al. Verification of consumers’ experiences and perceptions of genetic discrimination and its impact on utilization of genetic testing. Genet Med 2009; 11: 193-201.
- 8. Warner BJ, Curnow LJ, Polglase AL, Debinski HS. Factors influencing uptake of genetic testing for colorectal cancer risk in an Australian Jewish population. J Genet Couns 2005; 14: 387-394.
- 9. Müller A, Fishel R. Mismatch repair and the hereditary non-polyposis colorectal cancer syndrome (HNPCC). Cancer Invest 2002; 20: 102-109.
- 10. Loeb LA. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res 1991; 51: 3075-3079.
- 11. Dunlop MG, Farrington SM, Nicholl I, et al. Population carrier frequency of hMSH2 and hMLH1 mutations. Br J Cancer 2000; 83: 1643-1645.
- 12. Jenkins MA, Baglietto L, Dowty JG, et al. Cancer risks for mismatch repair gene mutation carriers: a population-based early onset case-family study. Clin Gastroenterol Hepatol 2006; 4: 489-498.
- 13. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet 2005; 42: 491-496.
- 14. Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006; 296: 1507-1517.
- 15. Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000; 118: 829-834.
- 16. Southey MC, Jenkins MA, Mead L, et al. Use of molecular tumor characteristics to prioritize mismatch repair gene testing in early-onset colorectal cancer. J Clin Oncol 2005; 23: 6524-6532.
- 17. Codori AM, Petersen GM, Miglioretti DL, et al. Attitudes toward colon cancer gene testing: factors predicting test uptake. Cancer Epidemiol Biomarkers Prev 1999; 8: 345-351.
- 18. Meiser B. Psychological impact of genetic testing for cancer susceptibility: an update of the literature. Psychooncology 2005; 14: 1060-1074.
- 19. Aktan-Collan K, Mecklin JP, Järvinen H, et al. Predictive genetic testing for hereditary non-polyposis colorectal cancer: uptake and long-term satisfaction. Int J Cancer 2000; 89: 44-50.
- 20. Hadley DW, Jenkins J, Dimond E, et al. Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Arch Intern Med 2003; 163: 573-582.
- 21. Lerman C, Marshall J, Audrain J, Gomez-Caminero A. Genetic testing for colon cancer susceptibility: anticipated reactions of patients and challenges to providers. Int J Cancer 1996; 69: 58-61.
- 22. UK Department of Health, Association of British Insurers. Concordat and moratorium on genetics and insurance. London: DH, 2005.
- 23. Investment and Financial Services Association. IFSA Standard No. 11. Genetic testing policy. Sydney: IFSA, 2005. http://www.ifsa.com.au/documents/IFSA%20Standard%20No%2011.pdf (accessed Jun 2009).





Abstract
Objective: To assess whether knowledge of insurance implications influenced uptake of genetic testing by participants in a research study of the causes of colorectal cancer.
Design, setting and participants: Analysis of uptake of genetic testing by participants in the population-based Victorian Colorectal Cancer Family Study during two periods: from 1999 to 2003, when participants were not informed of any potential effect of genetic testing conducted during the study on their eligibility for new insurance policies; and from 2003 to 2006, when the protocol was changed to provide participants with information on the potential effect of genetic testing on insurance eligibility.
Main outcome measure: Uptake of genetic testing for germline mutations in DNA mismatch repair (MMR) genes at a family cancer clinic.
Results: The proportion of participants who declined genetic testing among those informed of insurance implications was more than double the proportion among those without this knowledge (29/59 [49%] v 9/47 [19%]; P = 0.002). This difference could not be explained statistically by adjusting for measured putative predictors.
Conclusion: Identification of people with a mutation in an MMR gene has clinical importance, and such screening may be a cost-effective way to reduce the burden of colorectal cancer in the community. If people are choosing not to obtain genetic information because of how it will affect their eligibility for insurance, reforms to existing insurance practices are indicated.