Domiciliary oxygen therapy (DOT) involves providing oxygen equipment for ongoing home use. It is prescribed as long-term oxygen therapy (at least 15 hours a day) for chronic hypoxaemia, or to prevent oxygen desaturation associated with exercise or sleep.1,2 DOT provides mortality and morbidity benefits for patients with chronic hypoxaemia caused by chronic obstructive pulmonary disease (COPD),1,3-5 but it constitutes a prolonged commitment of resources to individual patients. A study from one Australian state found a mean survival of 2.9 years for patients with COPD who were prescribed DOT, but other countries have reported longer survival times.6 By extrapolation from prevalence and cost data in one state, DOT in Australia was estimated to cost $30.4 million in 2001.7
DOT is funded by all Australian states and territories (Box 1), and two federal government departments: the Department of Veterans’ Affairs (DVA) and the Department of Health and Ageing (DoHA). The DVA funds clinically eligible war veterans,8 and the DoHA pays oxygen subsidies to federally funded, residential aged care facilities.9
The position statement Adult domiciliary oxygen therapy by the Thoracic Society of Australia and New Zealand provides an evidence base for DOT services.1 Service provision is reported to vary between jurisdictions, but the extent and impact of this is unclear.1,7 The many funding sources and departments administering DOT programs make it difficult to assess accurately the rate of prescription and the total cost of these programs in Australia.
For each jurisdiction, the average cost per patient per year was derived by dividing the total cost of DOT prescriptions by the number of patients, and patient numbers and costs were summed to calculate national DOT data. Projected demographic data for June 2006, published in 2003 by the Australian Bureau of Statistics, were used for population-based results.10
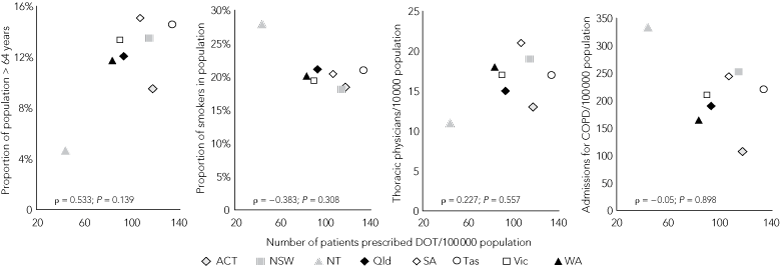
Associations between the prescription rate of DOT and the following variables in each state were tested using the Spearman rank correlation coefficient, rho (ρ): proportion of the population older than 64 years; proportion of the population with an accessibility–remoteness index of 0–2.4 (ie, living in inner regional areas or cities); admission rates for COPD per 100 000 population (International classification of diseases, 10th revision [ICD-10], code J44); smoking rates in those aged > 14 years; and number of practising thoracic physicians per 10 000 population.11-14
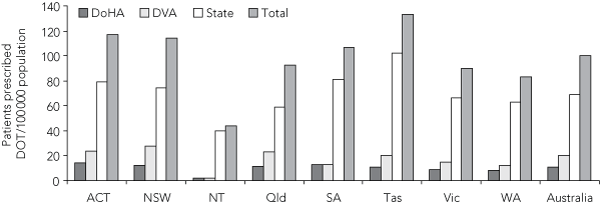
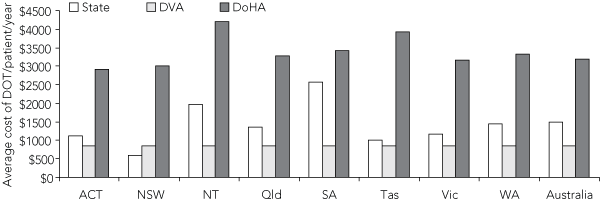
In 2005, Australian governments funded DOT for 20 127 patients (100 patients per 100 000 population) through 59 different services, at a cost of over $31 million (Box 2). Box 3 lists the number of patients prescribed DOT within each state or territory and in Australia as a whole, by the source of funding. Prescription rates for DOT per 100 000 population within each state ranged from 44 (NT) to 133 (Tas), a threefold difference (Box 4). Costs of DOT per patient prescribed per year funded by individual states and territories (excluding NSW, in which cost data underestimated expenditure) ranged from $1014 in Tas to $2574 in SA (Box 5).
DOT prescription rates in the states or territories were not associated with the population age profile; smoking rates; access to thoracic physicians; or admission rates for COPD (all P > 0.05 [Box 6]); nor with the proportion of the population living in cities or inner regional areas (ρ = 0.317; P = 0.4).
State governments used two broad funding models (Box 7). Centralised services (a single body administering the program) were used in the ACT, metropolitan WA, Qld and the NT. In the other states, funds were distributed to regional health authorities, or to aids and equipment programs aligned with health areas.
All services provided concentrators for home use. Portable oxygen was funded in all states except Qld (where it was limited to children and patients waiting for heart or lung transplants). An exercise test showing the benefit of ambulatory oxygen was required in Tas, Vic, Qld, NSW and WA. Patients in NSW were required to pay for oxygen refills. Funding for refills (delivery of new cylinders) varied between and within some states with decentralised services (Box 7). Liquid oxygen was used in SA to a limited extent (patients with high portability needs) at a cost of $230 per month.
The DVA funded any oxygen equipment indicated by the prescriber, including unlimited cylinder refills and deliveries for eligible veterans. The DoHA paid a monthly oxygen equipment subsidy via Medicare directly to residential aged care facilities for each resident prescribed DOT.9
The total number and rate of DOT prescriptions in Australia, including data from the DVA, the DoHA and individual states and territories, have not been published previously. Our data for the number of patients whose DOT was state-funded, and the costs incurred, were lower than previous estimates. Jones et al extrapolated 2002–2004 data from Tas to arrive at an estimate of 21 000 patients using state-funded DOT, exceeding our result by 59%.15 This difference is consistent with our finding that Tas had the highest rate of prescription of DOT.
Previous cost estimates for all state-funded services exceed ours by 46%–51%.7,15 Crockett et al estimated the total cost for Australia to be $30.4 million in 2001 by extrapolating data on DOT prescriptions from one DOT service in SA to the whole of Australia (111 patients prescribed DOT per 100 000 population, costing $1400 per patient per year).7 Our study found a lower state-funded rate in SA (83 per 100 000) and a higher cost per patient per year of $2574. This may be due to cost variability between services in SA, and the administrative costs included in our data from the Department of Health, SA. The prescription rate in Tas for DOT in 2002–2004 was found to be 102 per 100 000 population (comparable with our finding) at a cost of $1498 per patient per year (which extrapolates to a national figure of $31.5 million).15 While both these cost estimates approximate our findings, neither included data from the DVA or the DoHA. This highlights the potential for error when extrapolating, and the benefit that a national oxygen database would provide.
Our estimated national cost for DOT does not include administrative costs incurred by the DoHA and the DVA, nor the indirect clinical costs associated with assessment, reassessment, education and support of patients prescribed DOT. This represents a significant underestimation of the real cost of DOT. Appropriate assessment of patient suitability is needed to avoid unnecessary prescriptions, but requires clinician time (physiotherapist, physician, nurse) and resources (arterial blood gas analysis, overnight oximetry). Without this, poor adherence to prescribed DOT may negate benefit and waste expenditure.16
The lack of consistent definitions of data makes it difficult to interpret interstate variability. Factors that may influence cost include equipment type, delivery distance and frequency, and market competition. Some NSW state DOT services owned oxygen concentrators. Surprisingly, not all states with liberal cylinder refills (Box 7) incurred higher costs (costs were lowest in the ACT and Vic). More equitable provision of DOT may therefore be achievable.
Rates of DOT prescription varied widely between states. Those with less urbanised populations and arguably less access to medical services may be expected to show lower rates of DOT, as was seen in the NT (44 per 100 000). However, we were unable to show an association between remoteness and prevalence of DOT prescription within each state using grouped state-level data. Regional variations in DOT prescription rates in Tas, and higher rates where access to respiratory specialists was limited, has been reported previously.15 The NT data may be explained by the higher percentage of this population identifying as Indigenous and their increased prevalence of smoking. More NT patients may have benefited from DOT but, as smokers, they would have been ineligible.7,17
COPD was the most frequent diagnosis,1,5,6,15 representing 57% of patients receiving DOT from the Medical Aids Subsidy Scheme in Qld in 2005, and 48% of patients prescribed DOT in Tas.15 Geographical heterogeneity in the prevalence or severity of COPD could explain the variation in DOT usage; however, we did not find this association in state data. Variability in rates of DOT prescription between Danish counties was not explained by differences in COPD admissions, a crude surrogate for severe COPD.18 Other factors that may have influenced variability in Australia include the means test for eligibility in NSW and Qld, differing reassessment requirements, and variable survival of patients (in part due to poor adherence).
National DOT schemes operate in France, Sweden and, more recently, in England and Wales, with the capacity to benchmark rates of use, cost, clinical outcomes, access and adherence to guidelines.1,19-22 The number of patients prescribed DOT in England and Wales (76 763) was first reported in 2007 after the National Health Service implemented a national oxygen service.19
2 Domiciliary oxygen therapy (DOT) services in Australia, patients prescribed DOT, and costs, by source of funding (2005)
3 Number of patients prescribed domiciliary oxygen therapy in Australian states and territories, by source of funding (2005)
DVA = Department of Veterans’ Affairs. DoHA = Department of Health and Ageing. | |||||||||||||||
4 Prescription rates per 100 000 population for domiciliary oxygen therapy (DOT) in Australian states and territories, by source of funding (2005)
 | |||||||||||||||
|
DoHA = Department of Health and Ageing. DVA = Department of Veterans’ Affairs. | |||||||||||||||
5 Average cost of domiciliary oxygen therapy (DOT) per patient prescribed per year in Australian states and territories, by source of funding (2005)
 | |||||||||||||||
6 Association between prescription rates of domiciliary oxygen therapy (DOT) in Australian states and territories and demographic variables
 | |||||||||||||||
- John G Serginson1
- Ian A Yang1,2
- John G Armstrong3,2
- David M Cooper4,2
- Anthony M Matthiesson5
- Stephen C Morrison6
- Judy M Gair7
- Barbara Cooper8
- Paul V Zimmerman1,2
- 1 Thoracic Program, Department of Thoracic Medicine, The Prince Charles Hospital, Brisbane, QLD.
- 2 School of Medicine, University of Queensland, Brisbane, QLD.
- 3 Department of Respiratory and Sleep Medicine, Princess Alexandra Hospital, Brisbane, QLD.
- 4 Department of Respiratory and Sleep Medicine, Mater Children’s Hospital, Brisbane, QLD.
- 5 Department of Respiratory Medicine, Townsville General Hospital, Townsville, QLD.
- 6 Department of Thoracic and Sleep Medicine, Royal Brisbane and Women’s Hospital, Brisbane, QLD.
- 7 Rehabilitation Appliances Program, Department of Veterans’ Affairs, Brisbane, QLD.
- 8 Medical Aids Subsidy Scheme, Brisbane, QLD.
Data were contributed by: DoHA; Medicare Australia; DVA; Aids and Equipment Program, Department of Human Services, Vic; ACT Equipment Scheme, ACT; Territory Independence and Mobility Equipment Scheme, NT; Program of Appliances for Disabled People, NSW Health; Acute Care and Clinical Services, SA Health; Clinical Policy Branch, WA Health; Silver Chain Oxygen Service, Perth; Royal Darwin Hospital; Prince of Wales Hospital; Wagga Wagga Hospital; St George Hospital; Launceston General Hospital; Burnie Hospital; Royal Hobart Hospital; Royal Children’s Hospital; Melbourne Hospital; The Alfred Hospital; St Vincent’s Hospital, Melbourne; Women’s and Children’s Hospital; Lyell McEwin Hospital; Modbury Hospital; Royal Adelaide Hospital; Flinders Medical Centre; The Queen Elizabeth Hospital; and Air Liquide Healthcare.
The Medical Aids Subsidy Scheme (MASS), which provides the state-funded DOT service in Qld, funded and conducted the review, when reviewing its own services. Judy Gair is employed in the DVA Rehabilitation Appliances Program. All other authors are members of the MASS Oxygen Clinical Advisory Committee.
- 1. McDonald CF, Crockett AJ, Young I. Adult domiciliary oxygen therapy. Position statement of the Thoracic Society of Australia and New Zealand. Med J Aust 2005; 182: 621-626. <MJA full text>
- 2. Law S, Lehoux P. Hospital technology at home: portable oxygen therapy in COPD. Montreal: Agence d’Evaluation des Technologies et des Modes d’Intervention en Sante (AETMIS), 2004.
- 3. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease. Ann Intern Med 1980; 93: 391-398.
- 4. Report of the Medical Research Council Working Party. Long-term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981; 1: 681-686.
- 5. Crockett AJ, Cranston JM, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2000; (4): CD001744.
- 6. Cranston JM, Nguyen AM, Crockett AJ. The relative survival of COPD patients on long-term oxygen therapy in Australia: a comparative study. Respirology 2004; 9: 237-242.
- 7. Crockett AJ, Cranston J, Moss J. Chronic obstructive pulmonary disease (COPD). Economic case statement. Brisbane: The Australian Lung Foundation, 2002: 17. http://www.lungfoundation.com.au/images/stories/docs/COPD_Economic_Case_Statement-web-small.pdf (accessed Sep 2008).
- 8. Australian Government Department of Veterans’ Affairs. Rehabilitation Appliances Program. DVA procedural guidelines for home medical oxygen therapy prescribers. http://www.dva.gov.au/service_providers/rap/Documents/oxygenguidelines.doc (accessed Sep 2009).
- 9. Australian Department of Health and Ageing. Residential care manual. http://www.health.gov.au/internet/main/publishing.nsf/Content/ageing-manuals-rcm-rcmindx1.htm (accessed Sep 2009).
- 10. Australian Bureau of Statistics. Australian social trends, 2003. Canberra: ABS, 2003. (ABS Cat. No. 4102.0.)
- 11. Australian Bureau of Statistics. Outcomes of ABS views on remoteness consultation, Australia. Canberra: ABS, 2001. (ABS Cat. No. 1244.0.00.001.)
- 12. Australian Institute of Health and Welfare. Australian hospital statistics 2004–05. Health Services Series No. 26. Canberra: AIHW, 2006. (AIHW Cat. No. HSE 41.)
- 13. Australian Medical Workforce Advisory Committee. The specialist thoracic medicine workforce in Australia. Supply and requirements 1999–2010. Sydney: AMWAC, 2000. (AMWAC Report 2000.1.)
- 14. Scollo MM, Winstanley MH, editors. Tobacco in Australia: facts and issues. 3rd ed. Melbourne: Cancer Council Victoria, 2008.
- 15. Jones A, Wood-Baker R, Walters EH. Domiciliary oxygen therapy services in Tasmania: prescription, usage and impact of a specialist clinic. Med J Aust 2007; 186: 632-634. <MJA full text>
- 16. Cullen DL. Long term oxygen therapy adherence and COPD: what we don’t know. Chron Respir Dis 2006; 3: 217–222.
- 17. Australian Bureau of Statistics. Population characteristics, Aboriginal and Torres Strait Islander Australians, Northern Territory, 2006. Canberra: ABS, 2008. (ABS Cat. No. 4713.7.55.001.)
- 18. Ringbaek TJ, Lange P, Viskum K. Geographic variation in long-term oxygen therapy in Denmark: factors related to adherence to guidelines for long-term oxygen therapy. Chest 2001; 119: 1711-1716.
- 19. Donaldson GC, Edmonds G, Balfour-Lynn I, et al. Development of the British Thoracic Society home oxygen database and prevalence of home oxygen use in England and Wales. Thorax 2007; 62 (Suppl 3): A64. http://thorax.bmj.com/cgi/content/full/62/Suppl_3/A64 (accessed Sep 2009).
- 20. Ringbaek T, Lange P. The impact of the Danish Oxygen Register on adherence to guidelines for long-term oxygen therapy in COPD patients. Respir Med 2006; 100: 218-225.
- 21. Strom K, Boe J. A national register for long-term oxygen therapy in chronic hypoxia: preliminary results. Eur Respir J 1988; 1: 952-958.
- 22. Chailleux E, Fauroux B, Binet F, et al. Predictors of survival in patients receiving domiciliary oxygen therapy or mechanical ventilation. A 10-year analysis of ANTADIR Observatory. Chest 1996; 109: 741-749.





Abstract
Objectives: To determine the rate of prescription of and government expenditure for domiciliary oxygen therapy (DOT) in Australia, and to identify interstate differences in rates, costs and service provision.
Design: Retrospective observational study.
Participants and setting: Government departments and health services (state and federal) that funded DOT in Australia in the 2004–05 financial year (including the Department of Veterans’ Affairs [DVA] and the Department of Health and Ageing [DoHA]).
Main outcome measures: Prescription rates, cost of DOT in 2004–05, and services provided in each jurisdiction.
Results: In 2005, 20 127 patients were using DOT, giving a national prevalence of 100 prescriptions per 100 000 population. The total cost was about $31 million. State governments, the DVA and the DoHA funded 13 899 (69%), 4084 (20%) and 2144 (11%) patients, respectively. Prescription rates varied threefold between the states, ranging from 44 (Northern Territory) to 133 (Tasmania) per 100 000 population. Cost per patient per year varied fourfold between the DVA and the DoHA. All jurisdictions funded oxygen according to the clinical criteria of the Thoracic Society of Australia and New Zealand, but considerable variability in service provision was identified.
Conclusion: DOT prescription rates and costs vary considerably between jurisdictions. An urgently needed national DOT register would enable the current variability to be understood and allow service planning and benchmarking of clinical outcomes.