In the 1960s, ischaemic heart disease (IHD) was the most common cause of death among Australians, accounting for a quarter of all deaths.1 Since then, IHD death rates have fallen an average of 3.6% every year for men and 3.0% for women.1,2 This fall has been attributed, in part, to a decrease in IHD incidence, largely a result of reduced tobacco use.3,4 Better survival rates with improved treatment have also been cited as a factor,3 but there are no national estimates of trends in IHD survival rates.
As in Australia as a whole, IHD mortality decreased in the Northern Territory over the past two decades, but this decrease was confined to the non-Indigenous population.5 In contrast, deaths from IHD in the Indigenous population increased by 5.7% annually between 1977 and 1990, and continued to increase after 1990, albeit at a slower rate of 1.1% annually, to 2001.6
The reasons for this difference are not certain. The IHD incidence rate among Indigenous Australians was calculated for 2002–2003 as three times higher than that in the general population.7 However, long-term national data on IHD incidence and survival for Indigenous people are lacking.
Ischaemic heart disease includes AMI, angina pectoris and other related conditions. However, the difficulty of obtaining reliable data on angina pectoris and related conditions means that monitoring of incidence and survival is necessarily confined to AMI events.4,8
We used deaths data from the Australian Bureau of Statistics (ABS) to identify all deaths of NT residents from AMI that occurred in Australia between 1 January 1992 and 31 December 2004. All deaths with an underlying cause of IHD (ICD-9 codes 410–414 for 1992–1996; ICD-10 codes I20–I25 for 1997–2004) were included. These expanded codes were necessary as the distinction between AMI and other IHD subclassifications in deaths data has been shown to be unreliable.8 This approach was consistent with that used in previous studies of national AMI incidence.4,7
Combining hospital inpatient and ABS death data created an overlapping list of AMI cases. To eliminate the overlap, we matched individuals identified in the hospital data with the National Death Index (NDI) held at the Australian Institute of Health and Welfare using probabilistic matching on names, date of birth and sex.9 Potential matches were verified manually. We then separated matched cases from the IHD deaths identified from the ABS data using the death registration number, to establish a list of new AMI patients who died without hospital admission.
We identified 3419 new AMI cases in NT residents between 1 January 1992 and 31 December 2004 (Box 1). Of these, 1417 people (41%) were Indigenous, and 2414 (71%) were men. Median age was older for women than for men, and also for non-Indigenous than for Indigenous people.
The univariate analysis showed that the age-adjusted AMI incidence rate was higher for Indigenous than for non-Indigenous people, higher for men than for women, and higher in remote areas than in urban areas for non-Indigenous people, but not for Indigenous people (Box 2). Incidence increased sharply by age group.
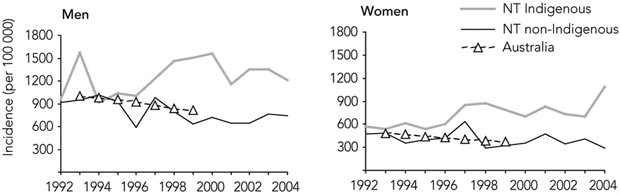
National AMI incidence has been previously reported for the population aged 40 years or over.3 In this age group in the NT, the AMI incidence rate for non-Indigenous people dropped between 1992 and 2004 by 23% (IRR, 0.98 per year; 95% CI, 0.95–1.01). In the years for which national data are available, AMI incidence rates for non-Indigenous NT men and women did not differ greatly from national rates, and decreases were at much the same rate (Box 3).
In contrast, the incidence rates among Indigenous men and women aged 40 years or over in the NT, although similar to NT non-Indigenous and national rates at the beginning of the study period, increased between 1992 and 2004 by 48% (IRR, 1.03 per year; 95% CI, 1.00–1.07). By 1998, these rates were about twice those in the corresponding national populations (Box 3).
After adjustment for risk factors (Box 4), AMI incidence was 56% lower in women than in men. For non-Indigenous people, incidence increased with age by 10% per year, was 102% higher in remote than urban areas, and decreased by 20% between 1992 and 2004 (IRR, 0.98; 95% CI, 0.97–1.00). For Indigenous people, incidence increased with age by 6% per year (1.06; 95% CI, 1.05–1.06), was similar in urban and remote areas (IRR, 1.05; 95% CI, 0.93–1.21), and increased by 60% between 1992 and 2004 (IRR, 1.04 per year; 95% CI, 1.02–1.06).
The IRR for Indigenous compared with non-Indigenous people varied by age at diagnosis, year of diagnosis and residence (Box 4). Compared with the non-Indigenous urban population, at the median age of AMI diagnosis (58 years) Indigenous AMI incidence was 95% higher in 1992 (IRR, 1.95; 95% CI, 1.60–2.37) and 290% higher in 2004 (IRR, 3.90; 95% CI, 3.27–4.65).
The proportion of deaths from AMI that occurred without hospital admission (pre-hospital deaths) was lower in women than in men (OR, 0.75) and increased with age (OR, 1.03 per year of age) (Box 4). The proportion was also higher in Indigenous than in non-Indigenous people (OR, 1.88, at median age of diagnosis [58 years] and middle year of study period [1998]).
For patients admitted to hospital, the death rate after an AMI was higher for Indigenous than for non-Indigenous patients (HR, 1.75 at median age of diagnosis and middle year of study period) (Box 4), but decreased for both groups between 1992 and 2004, by 75% for non-Indigenous people and 50% for Indigenous people (HR, 0.94 per year; 95% CI, 0.90–0.99). The death rate for hospitalised patients did not differ by sex or by remoteness of residence, but did increase with age at diagnosis, by 7% per year for non-Indigenous patients and 3% per year for Indigenous patients (HR, 1.03; 95% CI, 1.02–1.05).
For all people with AMI (ie, with and without hospital admission), the death rate was higher for Indigenous than for non-Indigenous people (HR, 1.44; 95% CI, 1.21–1.70) at median age at diagnosis for urban cases (Box 4). The death rate was 11% lower for women than men, and decreased by 29% between 1992 and 2004. For non-Indigenous people, the death rate increased with age at diagnosis by 3% per year, and was 27% higher for remote than urban residents. For Indigenous people, the death rate increased with age at diagnosis by 2% per year (HR, 1.02; 95% CI, 1.01–1.02) and was similar for remote and urban residents (HR, 1.06; 95% CI, 0.90–1.25).
We found that annual incidence of AMI in the non-Indigenous population of the NT was similar to that in the general Australian population between 1993 and 1999, and decreased at a similar rate during this time.4 During our study period, 1992–2004, there was also a considerable increase in survival rates for patients hospitalised with AMI. Increased survival is consistent with the growth of specialised coronary care services and the growing emphasis on post-hospital management of patients with AMI. However, offsetting this improvement was our finding that the risk of pre-hospital death changed little, so that the improvement in non-Indigenous all-cases survival was smaller than that in hospitalised patients (death rates decreased by 29% and 75%, respectively).
From these results, we conclude that the previously reported rising rate of deaths associated with IHD5,6 in the NT Indigenous population is due to increased incidence of IHD in this population, moderated by the effect of improved survival rates.
Many factors contribute to the poorer outcomes observed for the Indigenous NT population after first hospitalisation for AMI. These include higher rates of AMI-related risk factors, and poorer access to coronary procedures that improve outcomes.10,11 Access to procedures is itself influenced by the individual’s preference and consent to treatment, geographic remoteness and clinical decision making.12 The rate of coronary procedures has been reported as being lower for Indigenous than non-Indigenous people, not only during the index admission but also during subsequent admissions.10 Other factors that influence outcome include delays to hospital presentation,13 affordability of medications, compliance with clinical management plans and access to primary care services.14
Our study had several limitations. Retrospective linking of hospital and deaths data provided a reliable, but not perfect, data source.9,15-20 For example, 40 of 667 patients (6%) known from hospital data to have died were not identified as deceased when matched to the NDI. Data may also have been duplicated for patients with more than one hospital admission and a variation in their identification details.
A 2003 study in Western Australia showed the importance of a clearance period in data collection, reporting a 13% overestimation of AMI incidence in the early years of studies that did not use such a period.21 We incorporated a 1.5-year clearance period into our study, which maximised the availability of hospital cases within the available dataset, but a small proportion of previous AMI admissions may have been overlooked.
Our study demonstrates the usefulness of routinely collected data for investigating population health trends and health system performance that previously could not be measured, particularly on a systemwide basis. The national study of AMI incidence and case fatality was not able to measure survival rates, as data for individuals could not be linked.4
In this study, the availability of unique patient identifiers across all NT public hospitals allowed the separate calculation of the proportion of people who died without hospital admission, and survival rates for hospitalised patients. These data provide important measures of health system performance, including rates of access to acute care (proportion who died without hospital admission), health service performance (survival for hospitalised patients) and an integrated measure of “whole system” performance (survival for all people after AMI).22 The different patterns of change over time for Indigenous and non-Indigenous people with AMI highlight the different issues that need to be addressed in each population group.
1 Demographic characteristics of people with new acute myocardial infarction, Northern Territory, 1992–2004, by Indigenous status
2 Age-adjusted incidence rate (95% CI) of acute myocardial infarction, Northern Territory, 1992–2004 (per 100 000 population)*
3 Incidence of acute myocardial infarction in populations aged ≥ 40 years in the Northern Territory (1992–2004), and in Australia (1993–1999)
Received 19 December 2007, accepted 27 October 2008
- Jiqiong You1
- John R Condon2
- Yuejen Zhao1
- Steven Guthridge1
- 1 Health Gains Planning, Department of Health and Community Services, Darwin, NT.
- 2 Menzies School of Health Research, Institute of Advanced Studies, Charles Darwin University, Darwin, NT.
We acknowledge the acute care staff and data warehouse team of the NT Department of Health and Community Services for their assistance with data access; and the National Death Index team at the Australian Institute of Health and Welfare for data matching.
None identified.
- 1. d’Espaignet ET. Trends in Australian mortality: disease of the circulatory system, 1950-1991. Canberra: Australian Institute of Health and Welfare, 1993.
- 2. Australian Institute of Health and Welfare. Australia’s health 2000: the seventh biennial health report of the Australian Institute of Health and Welfare. Canberra: AIHW, 2000.
- 3. Australian Institute of Health and Welfare. Heart, stroke and vascular diseases — Australian facts 2004. Canberra: AIHW and National Health Foundation of Australia, 2004.
- 4. Mathur S. Epidemic of coronary heart disease and its treatment in Australia. Canberra: Australian Institute of Health and Welfare, 2002.
- 5. Li SQ, Guthridge SL. Mortality in the Northern Territory, 1981-2000. Darwin: Department of Health and Community Services, 2005.
- 6. Thomas DP, Condon JR, Anderson IP, et al. Long-term trends in Indigenous deaths from chronic diseases in the Northern Territory: a foot on the brake, a foot on the accelerator. Med J Aust 2006; 185: 145-149. <MJA full text>
- 7. Mathur S, Moon L, Leigh S. Aboriginal and Torres Strait Islander people with coronary heart disease: further perspectives on health status and treatment. Canberra: Australian Institute of Health and Welfare, 2006.
- 8. Dobson A, Gibberd R, Leeder S. Death certification and coding for ischaemic heart disease in Australia. Am J Epidemiol 1983; 117: 397-405.
- 9. Coutinho ES, Coeli CM. [Accuracy of the probabilistic record linkage methodology to ascertain deaths in survival studies] [Portuguese]. Cad Saude Publica 2006; 22: 2249-2252.
- 10. Coory MD, Walsh WF. Rates of percutaneous coronary interventions and bypass surgery after acute myocardial infarction in Indigenous patients. Med J Aust 2005; 182: 507-512. <MJA full text>
- 11. Cunningham J. Diagnostic and therapeutic procedures among Australian hospital patients identified as Indigenous. Med J Aust 2002; 176: 58-62.
- 12. Cantor WJ, Goodman SG, Cannon CP, et al. Early cardiac catheterization is associated with lower mortality only among high-risk patients with ST- and non-ST-elevation acute coronary syndromes: observations from the OPUS-TIMI 16 trial. Am Heart J 2005; 149: 275-283.
- 13. Ong MA, Weeramanthri TS. Delay times and management of acute myocardial infarction in Indigenous and non-Indigenous people in the Northern Territory. Med J Aust 2000; 173: 201-204.
- 14. Rahimi AR, Spertus JA, Reid KJ, et al. Financial barriers to health care and outcomes after acute myocardial infarction. JAMA 2007; 297: 1063-1072.
- 15. Kelman C. The Australian National Death Index: an assessment of accuracy. Aust N Z J Public Health 2000; 24: 201-203.
- 16. Kariminia A, Butler T, Corben S, et al. Mortality among prisoners: how accurate is the Australian National Death Index? Aust N Z J Public Health 2005; 29: 572-575.
- 17. Magliano D, Liew D, Pater H, et al. Accuracy of the Australian National Death Index: comparison with adjudicated fatal outcomes among Australian participants in the Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) study. Aust N Z J Public Health 2003; 27: 649-653.
- 18. Powers J, Ball J, Adamson L, Dobson A. Effectiveness of the National Death Index for establishing the vital status of older women in the Australian Longitudinal Study on Women’s Health. Aust N Z J Public Health 2000; 24: 526-528.
- 19. Madsen M, Davidsen M, Rasmussen S, et al. The validity of the diagnosis of acute myocardial infarction in routine statistics: a comparison of mortality and hospital discharge data with the Danish MONICA registry. J Clin Epidemiol 2003; 56: 124-130.
- 20. Zingmond DS, Ye Z, Ettner SL, Liu H. Linking hospital discharge and death records — accuracy and sources of bias. J Clin Epidemiol 2004; 57: 21-29.
- 21. Brameld KJ, Holman CD, Lawrence DM, Hobbs MS. Improved methods for estimating incidence from linked hospital morbidity data. Int J Epidemiol 2003; 32: 617-624.
- 22. National Health Performance Committee. National report on health sector performance indicators 2003. Canberra: Australian Institute of Health and Welfare, 2004.





Abstract
Objective: To estimate the incidence and survival rates of acute myocardial infarction (AMI) for Northern Territory Indigenous and non-Indigenous populations.
Design and participants: Retrospective cohort study for all new AMI cases recorded in hospital inpatient data or registered as an ischaemic heart disease (IHD) death between 1992 and 2004.
Main outcome measures: Population-based incidence and survival rates by age, sex, Indigenous status, remoteness of residence and year of diagnosis.
Results: Over the 13-year study period, the incidence of AMI increased 60% in the NT Indigenous population (incidence rate ratio [IRR], 1.04; 95% CI, 1.02–1.06), but decreased 20% in the non-Indigenous population (IRR, 0.98; 95% CI, 0.97–1.00). Over the same period, there was an improvement in all-cases survival (ie, survival with and without hospital admission) for the NT Indigenous population due to a reduction in deaths both pre-hospital and after hospital admission (death rates reduced by 56% and 50%, respectively). The non-Indigenous all-cases death rate was reduced by 29% as a consequence of improved survival after hospital admission; there was no significant change in pre-hospital survival in this population. Important factors that affected outcome in all people after AMI were sex (better survival for women), age (survival declined with increasing age), remoteness (worse outcomes for non-Indigenous residents of remote areas), year of diagnosis and Indigenous status (hazard ratio, 1.44; 95% CI, 1.21–1.70).
Conclusions: Our results show that the increasing IHD mortality in the NT Indigenous population is a consequence of a rise in AMI incidence, while at the same time there has been some improvement in Indigenous AMI survival rates. The simultaneous decrease in IHD mortality in NT non-Indigenous people was a result of reduced AMI incidence and improved survival after AMI in those admitted to hospital. Our results inform population-specific strategies for a systemwide response to AMI management.