Warfarin is the predominant anticoagulant for protection against stroke in patients with atrial fibrillation and/or mechanical valve replacement. It is also used for management of venous thromboembolism. In Australia, warfarin use is rising at a rate of 9% per annum,1 with about 30% of patients commencing therapy being aged over 70 years.2 Despite its efficacy, warfarin remains a major contributor to potentially preventable medication-related adverse events.3,4 Annual rates of major bleeding range from 1% to 7%, with minor bleeding in 16% of patients with atrial fibrillation.5,6 A major determinant of bleeding is over-anticoagulation, as reflected by a high international normalised ratio (INR).
Previous research has focused on drug, genetic and patient factors known to increase risk of either sub- or supra-therapeutic response. However, it is increasingly recognised that system of care and qualitative factors such as medication compliance, cognition, warfarin knowledge, and social support7-10 have a major role in determining the safety of warfarin use.
Data were collected through structured interviews using both forced-response and open-ended questions. Patient interviews were conducted in their homes and focused on demographic and clinical characteristics, psychosocial risk factors, and ability to manage complex medication and understand issues relating specifically to warfarin. Patients were assessed with standardised measures including the Montreal Cognitive Assessment,11 the five-item Geriatric Depression Scale,12 Duke Social Support Index,13 Barthel Index of functional independence,14 Medication Regimen Complexity Index,15 and Morisky Medication Adherence Scale.16
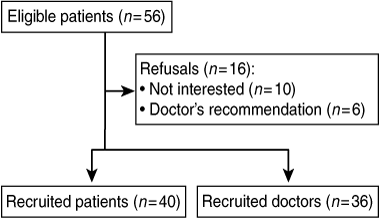
Forty of 56 eligible patients (response rate, 71%) and 36 of their 40 treating doctors (35 GPs and one specialist physician; response rate, 90%) were recruited (Box 1).
Twenty of the 36 doctors reported caring for more than 20 patients taking warfarin. All stated that specialists routinely initiate therapy and refer patients back to the GPs for ongoing management. Although many doctors (23/36) stated that they were comfortable in prescribing warfarin, more than a third (13) reported never making a unilateral decision to prescribe warfarin, preferring referral to a specialist (Box 2). All doctors provided routine prescriptions. Pathology services were used for monitoring and dosing by 32 doctors, primarily due to their time constraints. All doctors reported receiving timely notification of results from the pathology service.
Five of 36 doctors considered they had sole responsibility for routine management, 15 regarded management as involving a mutual relationship with the pathology service, and 16 deferred total responsibility to that service (Box 2). In contrast, most patients (33/40) regarded the pathology service to be their first port of call for any anticoagulation concerns. In instances requiring “after hours” medical intervention due to an elevated INR, 22 GPs expected to be collaboratively involved, while 14 had an understanding that the pathology service haematologist would contact the patient directly with the recommended intervention.
Demographic, clinical, cognitive and psychosocial characteristics of patients are shown in Box 3 and Box 4. These revealed that 30 of the 40 patients had multidimensional difficulties, including cognitive dysfunction, possible depression, and medication non-adherence. There was discordance between the doctors’ and researchers’ assessment of patients, with 12/36 doctors unaware of the measurable deficits in their patient’s cognition, mood and/or social connectedness. In addition, 25 doctors’ medication history records for their patients were inconsistent with the patients’ reported medication use, including absence of current prescription and complementary medications or retention of obsolete therapies in the record.
There is also a need for a more systematic approach to facilitate comprehensive assessment of individual risk, such as establishing a warfarin risk score. Validated bleeding-risk assessments, including CHADS217 and HEMORR2HAGES,18 consider numerous clinical characteristics but neglect psychosocial factors. We are currently developing a Warfarin Suitability Score that takes into account variables including contraindications, age, comorbid illnesses, intellectual function, depression, social supports, complexity of medication regimens, and medication management capability. This score may improve recognition of patients for whom treatment is more likely to be problematic and who may require assistance with warfarin management.
When high-risk patients are identified, the appropriate model of shared care should be considered. Responsibility may best be confined to a single provider who can explore suitable measures, including simplifying medication regimens and enlisting family help and the assistance of pharmacists or other agencies. A practical approach to service provision would be for the GP to take on this role. Interviews highlighted doctors’ time constraints as a factor that made the efficiency and reliability of the pathology service an attractive option. Time limitations are recognised as a barrier to the holistic care of elderly complex patients.19,20 One solution may be to delegate responsibility for education and follow-up of high-risk patients to a practice nurse, rather than referral to an external pathology provider. Patient education currently provided by the participating pathology service includes written information regarding warfarin at the time of initiation and an understanding of the service provided. Appropriate scheduled remuneration of nurses and/or pathology services may improve existing, and facilitate future, quality care systems.
Division of responsibility for providing education must be clear. Although it is plausible that education was provided, almost half the patients in our study perceived they had never received any warfarin education — the majority reported no regular discussion with their doctor, and most showed measurable ignorance about anticoagulation. The possibility of recall bias in the patients as a result of cognitive deficits cannot be excluded. The contribution of insufficient education and knowledge to poor anticoagulation control has been demonstrated,21-23 and patient report of absence of medication information has been identified as an independent predictor of future adverse events.24
Our study is limited by absence of a control arm to assess psychosocial deficits in patients with stable anticoagulation levels. Patients commencing warfarin for atrial fibrillation are generally elderly2 and may be uniformly affected by cognitive and social variables. The relative contribution of these variables will be assessed in a subsequent case–control study by our group. Further, we can make no comparisons or assumptions about patients managed within public pathology services or independently by their primary care doctors, or whether the factors examined contribute to instability of anticoagulation in hospital inpatients or patients in residential care. The system of care discussed and our recommendations for change may not be generalisable to areas where private pathology providers do not assume a similar role (eg, in other Australian states where the service provides INR testing but not dosage) or where there are stated policies for warfarin management. A better study design would have been to randomly select patients from randomly selected GPs. However, although doctors in this sample were identified by their association with patients experiencing an episode of significant over-anticoagulation, they also managed patients whose anticoagulation levels were stable. This suggests their recorded experiences are likely to be representative of other practitioners.
2 Doctors’ experience with and approach to warfarin management
- Judy A Lowthian1
- Basia O Diug1
- Sue M Evans1
- Ellen L Maxwell2
- Alison M Street3
- Leon Piterman4
- John J McNeil4
- 1 NHMRC Centre of Research Excellence in Patient Safety, Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC.
- 2 Melbourne Pathology, Melbourne, VIC.
- 3 Alfred Health, Melbourne, VIC.
- 4 Monash University, Melbourne, VIC.
We would like to thank Melbourne Pathology for assistance with recruitment; and Dr Shiong Tan for assistance with development of interview questions. This project was funded as part of National Health and Medical Research Council (NHMRC) Project Grant No. 43763.
Ellen Maxwell has been Director of Haematology at Melbourne Pathology, the private pathology provider recruiting patients to this study, since 2003, with primary responsibility for the anticoagulation service.
- 1. Baker RI, Coughlin PB, Gallus AS, et al. Warfarin reversal: consensus guidelines, on behalf of the Australasian Society of Thrombosis and Haemostasis. Med J Aust 2004; 181: 492-497. <MJA full text>
- 2. Palareti G, Hirsh J, Legnani C, et al. Oral anticoagulation treatment in the elderly: a nested, prospective, case–control study. Arch Intern Med 2000; 160: 470-478.
- 3. Runciman WB, Roughead EE, Semple SJ, Adams RJ. Adverse drug events and medication errors in Australia. Int J Qual Health Care 2003; 15 Suppl 1: i49-i59.
- 4. Makeham MA, Saltman DC, Kidd MR. Lessons from the TAPS study. Warfarin: a major cause of threats to patient safety. Aust Fam Physician 2008; 37: 817-818.
- 5. Schulman S. Care of patients receiving long-term anticoagulant therapy. N Engl J Med 2003; 349: 675-683.
- 6. Connolly SJ, Laupacis A, Gent M, et al. Canadian Atrial Fibrillation Anticoagulation (CAFA) Study. J Am Coll Cardiol 1991; 18: 349-355.
- 7. Kimmel SE, Chen Z, Price M, et al. The influence of patient adherence on anticoagulation control with warfarin: results from the International Normalized Ratio Adherence and Genetics (IN-RANGE) Study. Arch Intern Med 2007; 167: 229-235.
- 8. Hutchison LC, Jones SK, West DS, Wei JY. Assessment of medication management by community-living elderly persons with two standardized assessment tools: a cross-sectional study. Am J Geriatr Pharmacother 2006; 4: 144-153.
- 9. Fang MC, Machtinger EL, Wang F, Schillinger D. Health literacy and anticoagulation-related outcomes among patients taking warfarin. J Gen Intern Med 2006; 21: 841-846.
- 10. Schauer DP, Moomaw CJ, Wess M, et al. Psychosocial risk factors for adverse outcomes in patients with nonvalvular atrial fibrillation receiving warfarin. J Gen Intern Med 2005; 20: 1114-1119.
- 11. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695-699.
- 12. Nguyen HV, Inderjeeth CA, Tang E, et al. Screening for depression in hospitalised and community-dwelling elderly: the use of the 4-item, 5-item and 15-item geriatric depression scales. Australas J Ageing 2006; 25: 204-208.
- 13. Goodger B, Byles J, Higganbotham N, Mishra G. Assessment of a short scale to measure social support among older people. Aust N Z J Public Health 1999; 23: 260-265.
- 14. Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988; 10: 61-63.
- 15. George J, Phun YT, Bailey MJ, et al. Development and validation of the Medication Regimen Complexity Index. Ann Pharmacother 2004; 38: 1369-1376.
- 16. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986; 24: 67-74.
- 17. Medi C, Hankey GJ, Freedman SB. Atrial fibrillation. Med J Aust 2007; 186: 197-202. <MJA full text>
- 18. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151: 713-719.
- 19. Yuen KJ, Behrndt MM, Jacklyn C, Mitchell GK. Palliative care at home: general practitioners working with palliative care teams. Med J Aust 2003; 179 (6 Suppl): S38-S40. <MJA full text>
- 20. Bruce DG, Paley GA, Underwood PJ, et al. Communication problems between dementia carers and general practitioners: effect on access to community support services. Med J Aust 2002; 177: 186-188. <MJA full text>
- 21. Kagansky N, Knobler H, Rimon E, et al. Safety of anticoagulation therapy in well-informed older patients. Arch Intern Med 2004; 164: 2044-2050.
- 22. Palareti G, Legnani C, Guazzaloca G, et al. Risk factors for highly unstable response to oral anticoagulation: a case–control study. Br J Haematol 2005; 129: 72-78.
- 23. Tang EO, Lai CS, Lee KK, et al. Relationship between patients’ warfarin knowledge and anticoagulation control. Ann Pharmacother 2003; 37: 34-39.
- 24. Metlay JP, Hennessy S, Localio AR, et al. Patient reported receipt of medication instructions for warfarin is associated with reduced risk of serious bleeding events. J Gen Intern Med 2008; 23: 1589-1594.





Abstract
Objective: To identify potential weaknesses in the system of managing warfarin therapy.
Design, participants and setting: A structured interview-based study of 40 community-dwelling patients taking warfarin and with an international normalised ratio ≥ 6.0 and 36 of their treating doctors (35 general practitioners and 1 specialist), conducted between July and November 2007. Patients all received services from and were recruited sequentially by a large, private metropolitan pathology provider in Melbourne.
Main outcome measures: Patients’ demographic, clinical, cognitive and psychosocial characteristics, warfarin knowledge, medication complexity and adherence; and doctors’ experience with, approach to and involvement in warfarin management, and their perception of responsibility for warfarin management and patient education.
Results: Interviews revealed multiple difficulties, including cognitive dysfunction, possible depression, and medication non-adherence, in 30 of 40 patients. Of 36 doctors interviewed, 12 were unaware of these difficulties in their patients. Five doctors considered they had sole responsibility for their patients’ anticoagulation, while 15 confirmed a mutual relationship with the pathology service, and 16 deferred total responsibility to the pathology provider. Only 14/36 doctors reported conducting patient education at commencement of warfarin therapy, with the other 22 stating this was the responsibility of the initiating specialist, pathology service or dispensing pharmacist.
Conclusions: There is a need for improved role clarification in coordinating warfarin management. We propose exploring the possibility of a Warfarin Suitability Score to assist better recognition of patients in whom treatment may be problematic, along with a model of care using practice nurses with GPs to facilitate optimal patient care.