The importance of normal maternal thyroid function in pregnancy and fetal development is well established.1-4 During the first trimester, the fetus is reliant on transplacental passage of maternal thyroxine, as the fetal thyroid is not fully functional until about 16 weeks’ gestation, whereas thyroid hormone receptors in fetal tissues are present and functional much earlier.1-4 Neuropsychomotor development is impaired and mean IQ scores are reduced in children born to women who had thyroid deficiency during pregnancy.5-7 Pregnancy complications such as spontaneous miscarriage and gestational hypertension are associated with maternal hypothyroidism. Rates of placental abruption and premature delivery are also increased with both maternal hypothyroidism and subclinical hypothyroidism.6,8,9
Several studies have attempted to derive pregnancy-specific reference ranges for thyroid function tests, with inconsistent results10-14 — perhaps reflecting differences in iodine status between studies and, in some studies, the inclusion of women with thyroid autoimmunity.10,11 No relevant studies have been conducted with Australian women. It has been suggested that, until trimester-specific and method-specific reference ranges are established, an upper limit for TSH in pregnant women of 2.5 mU/L (compared with 4.0–4.5 mU/L in non-pregnant women) should be adopted.15
We aimed to establish first-trimester-specific reference intervals for TSH, free thyroxine (fT4) and free triiodothyronine (fT3) in women in Western Australia. The iodine status of pregnant Western Australian women has not been specifically studied, but a recent study of school children in WA suggests it is an iodine-sufficient region.16
For each analyte, medians and the 2.5th and 97.5th percentiles were determined by the Harrell–Davis quantile estimator method17 for each week within the 9–13-week gestation range, and overall for the first trimester. For the purposes of determining reference intervals, women who were positive for TPOAb and/or TgAb were excluded. We also calculated 95% confidence intervals for the lower and upper limits of the first-trimester reference ranges, using the Harrell–Davis jackknife variance estimator.17 Shifts in the distribution of age, gestation and TSH, fT4 and fT3 between the antibody-positive and antibody-negative groups were assessed non-parametrically using Wilcoxon tests.
The study was approved by the Human Research Ethics Committee of Sir Charles Gairdner Hospital.
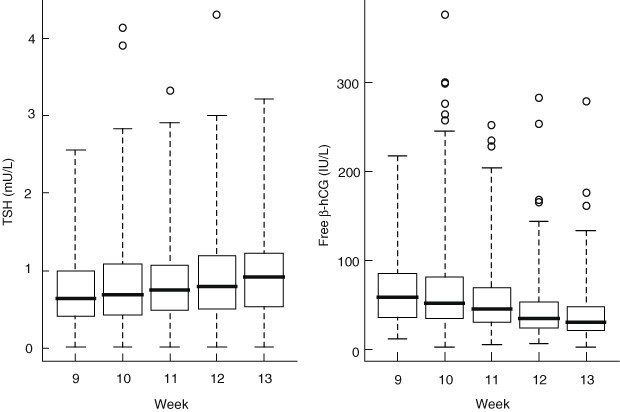
Of the remaining 2155 women, 338 (15.7%) were positive for TPOAb and/or TgAb. In the 1817 antibody-negative women, the median serum TSH concentration increased significantly across the 9–13-week gestation range (P = 0.020 from quantile regression analysis), whereas median serum free β-hCG concentrations decreased significantly over the same period (P < 0.001) (Box 1). There was a strong inverse relationship between TSH and free β-hCG (P = 0.002 from multiple regression analysis), which remained significant after adjustment for other statistically significant covariates (maternal age, week of gestation, fT4 and pregnancy-associated plasma protein A).
There was statistical evidence of a shift effect for TSH, fT4 and fT3 between the antibody-negative and antibody-positive groups of women (P < 0.001 for each comparison) (Box 2). There was no evidence of a difference in distribution of maternal age between antibody-negative and antibody-positive groups (P = 0.672). There was statistical evidence of a small shift in gestational age (P = 0.013); however, the magnitude of the mean difference was 1 day.
The reference group for deriving reference intervals comprised the 1817 antibody-negative women (maternal age range, 14.3–45.8 years; mean, 30.9 years) (Box 2). Reference ranges by gestation week for TSH, fT4 and fT3 derived from non-parametric analysis (with 95% confidence intervals for the upper and lower limits of the reference ranges) are shown in Box 3. For the whole reference group, the first-trimester-specific reference intervals were: TSH, 0.02–2.15 mU/L; fT4, 10.4–17.8 pmol/L; and fT3, 3.3–5.7 pmol/L. The TSH reference range is substantially lower than the established non-pregnant TSH reference range of 0.4–4.0 mU/L.
Our derived TSH reference interval of 0.02–2.15 mU/L is narrower than that reported by a study in the United States (0.01–4.05 mU/L).11 However, most of the women in that study were Hispanic, and there is evidence for differences between ethnic groups in thyroid function tests.18 Another US study also found a higher TSH upper limit of 3.61 mU/L, despite excluding TPOAb-positive women.12 In both of these studies,11,12 TSH was analysed on a different immunoassay platform using the DPC Immulite analyser (Diagnostic Products Corporation, Los Angeles, Calif, USA). In a recent US study of 585 thyroid disease-free and TPOAb-negative women of less than 14 weeks’ gestation, the derived TSH reference interval was 0.04–3.6 mU/L using the Bayer Advia Centaur analyser (Bayer Diagnostics, Tarrytown, NY, USA).13 In a study from Switzerland, researchers using the same automated immunoassay analyser as in our study (Abbott ARCHITECT) obtained a first-trimester-specific reference range for TSH in TPOAb- and TgAb-negative women of 0.09–2.83 mU/L.14 The higher upper limit in these studies13,14 may be due to the inclusion of women at earlier stages of gestation than in our study (less than 9 weeks), when the effects of hCG on the thyroid are not maximal. The diversity of reported first-trimester-specific reference intervals for thyroid function tests illustrates the importance of recruiting a local population and acknowledging methodological variations.
The strengths of our study include its large sample size, the inclusion of consecutively screened women from both metropolitan and regional areas, and the exclusion of women with thyroid autoimmunity in determining thyroid function test reference intervals. The prevalence of thyroid antibodies in our sample was similar to previous reports,6,12,14 suggesting that our sample was representative of pregnant women in general in this respect. None of the previous studies10-12 have evaluated iodine status in pregnant women. It has been documented that schoolchildren in WA are iodine-sufficient, based on the National Iodine Nutrition Study.16 Our assumption in this study that pregnant women in WA are also iodine-sufficient needs to be confirmed.
Our study shows that applying the conventional TSH reference interval to pregnant women results in misclassification of thyroid status in over 20% of women. Some 16% of women would have been erroneously classified as having subclinical or mild hyperthyroidism, which might lead to inappropriate investigation or treatment, and maternal anxiety. It has been shown that pregnant women with subclinical hyperthyroidism based on TSH at or below the 2.5th percentile are not at increased risk of adverse pregnancy outcomes.19
In a further 4.5% of the women, TSH was elevated according to the first-trimester-specific reference range; these women would not be identified with the conventional reference interval. More than half of these women (57%) tested positive for thyroid antibodies, suggesting they do have thyroid disease rather than being healthy outliers whose TSH concentrations happen to fall outside the reference range. The optimal management of these women is not known. In a recent randomised clinical trial, thyroxine replacement in pregnant women with thyroid antibodies and serum TSH within the conventional reference range (0.27–4.2 mU/L) was associated with a reduced rate of miscarriage and other pregnancy complications.20 On that basis, and until further data are available, it may be reasonable to offer thyroxine replacement therapy to women whose serum TSH concentration is above the upper limit of the first-trimester-specific reference interval, if thyroid antibodies are present.
Our study is timely, as there is growing debate about the merits of screening for thyroid dysfunction during pregnancy. Currently, there is controversy over whether universal screening or targeting of high-risk individuals is more appropriate.6,21 Whatever the outcome of that debate, it is likely that Australian pathology laboratories will receive increasing numbers of samples from pregnant women, and it is now appropriate for laboratories to move towards establishing trimester-specific (and method-specific) reference intervals for TSH, fT4, and fT3.
1 Serum thyrotropin (TSH) and free β-human chorionic gonadotropin (hCG) concentrations in 1817 antibody-negative women, by week of gestation
2 Demographic and thyroid function details for pregnant women at 9–13 weeks’ gestation*
R = reference group. TSH = serum thyrotropin. fT4 = free thyroxine. fT3 = free triiodothyronine. | |||||||||||||||
Received 5 February 2008, accepted 13 May 2008
- Rhonda M Gilbert1,2
- Narelle C Hadlow3
- John P Walsh1,4
- Stephen J Fletcher2
- Suzanne J Brown1
- Bronwyn G Stuckey1,4,5
- Ee Mun Lim1,2
- 1 Department of Endocrinology and Diabetes, Sir Charles Gairdner Hospital, Perth, WA.
- 2 Department of Clinical Biochemistry, PathWest QEII Laboratory, Perth, WA.
- 3 Department of Biochemistry and Cytogenetics, Western Diagnostic Pathology, Perth, WA.
- 4 School of Medicine and Pharmacology, University of Western Australia, Perth, WA.
- 5 Keogh Institute for Medical Research, Perth, WA.
We thank Abbott Diagnostics for donating the assay kits used in this study. We also thank Dr Peter O’Leary for supplying the data on first-trimester screening in WA.
None identified.
- 1. Lazarus JH. Thyroid hormones and neurodevelopment. Clin Endocrinol (Oxf) 1999; 50: 147-148.
- 2. Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999; 50: 149-155.
- 3. Vulsma T, Gons MH, de Vijlder JJ. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med 1989; 321: 13-16.
- 4. Morreale de Escobar G, Obregón M, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol 2004; 151 Suppl 3: U25-U37.
- 5. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341: 549-555.
- 6. Abalovich M, Amino N, Barbour LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2007; 92 (8 Suppl): S1-S47.
- 7. Pop VJ, Brouwers EP, Vader HL, et al. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003; 59: 282-288.
- 8. Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 2005; 105: 239-245.
- 9. Stagnaro-Green A, Chen X, Bogden JD, et al. The thyroid and pregnancy: a novel risk factor for very preterm delivery. Thyroid 2005; 15: 351-357.
- 10. Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 2001; 38: 329-332.
- 11. Dashe JS, Casey BM, Wells CE, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol 2005; 106: 753-757.
- 12. Haddow JE, Knight GJ, Palomaki GE, et al. The reference range and within-person variability of thyroid stimulating hormone during the first and second trimesters of pregnancy. J Med Screen 2004; 11: 170-174.
- 13. Pearce EN, Oken E, Gillman MW, et al. Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking, and multivitamin use. Endocr Pract 2008; 14: 33-39.
- 14. Stricker R, Echenard M, Eberhart R, et al. Evaluation of maternal thyroid function during pregnancy: the importance of using gestational age-specific reference intervals. Eur J Endocrinol 2007; 157: 509-514.
- 15. Mandel SJ, Spencer CA, Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid 2005; 15: 44-53.
- 16. Li M, Eastman CJ, Waite KV, et al. Are Australian children iodine deficient? Results of the Australian National Iodine Nutrition Study. Med J Aust 2006; 184: 165-169. <MJA full text>
- 17. Harrell FE, Davis CE. A new distribution-free quantile estimator. Biometrika 1982; 69: 635-640.
- 18. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87: 489-499.
- 19. Casey BM, Dashe JS, Wells CE, et al. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol 2006; 107: 337-341.
- 20. Negro R, Formoso G, Mangieri T, et al. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006; 91: 2587-2591.
- 21. Vaidya B, Anthony S, Bilous M, et al. Detection of thyroid dysfunction in early pregnancy: universal screening or targeted high-risk case finding? J Clin Endocrinol Metab 2007; 92: 203-207.





Abstract
Objective: To establish first-trimester-specific reference intervals for thyroid function tests in pregnant Australian women.
Design, setting and participants: Serum samples were collected from 2159 pregnant women (9–13 weeks’ gestation) attending a private pathology practice for first-trimester screening during October and November 2006. Levels of serum thyrotropin (TSH), free thyroxine (fT4), free triiodothyronine (fT3), thyroid peroxidase antibodies (TPOAb), and thyroglobulin antibodies (TgAb) were measured by chemiluminescent immunoassay (Abbott ARCHITECT analyser).
Main outcome measures: Reference intervals based on 2.5th and 97.5th percentiles for TSH, fT4 and fT3, after exclusion of 338 women with positive TPOAb or TgAb tests; comparison with reference intervals for non-pregnant women (TSH, 0.40–4.0 mU/L; fT4, 9–19 pmol/L; fT3, 3.0–5.5 pmol/L).
Results: Derived reference intervals for thyroid function tests during the first trimester of pregnancy were: TSH, 0.02–2.15 mU/L; fT4, 10.4–17.8 pmol/L; and fT3, 3.3–5.7 pmol/L. If the non-pregnant TSH reference range was applied to the study participants, 344 women (16.0%) whose serum TSH concentration was within the first-trimester-specific reference range would be misclassified as having subclinical hyperthyroidism, and 98 women (4.5%) with a TSH concentration above the first-trimester-specific upper reference limit would not be identified.
Conclusions: The reference interval for TSH during the first trimester of pregnancy differs substantially from that for non-pregnant women, and applying the general laboratory reference range to pregnant women results in misclassification of thyroid status for 20.5% of women. Australian pathology laboratories should adopt pregnancy-specific reference intervals for thyroid function tests.