Worldwide, primary liver cancer is the fifth most common cancer and the third most common cause of cancer-related death. In Australia, it is relatively uncommon, ranking 15th in males and 20th in females.1 However, the incidence and mortality of primary liver cancer have risen progressively over the past two decades; in New South Wales, primary liver cancer incidence rates have been rising faster than incidence rates of any other internal cancer.2
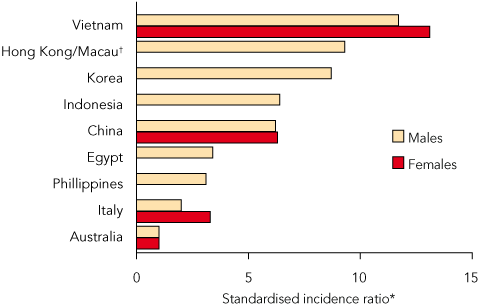
The Cancer Council NSW’s report Cancer incidence in New South Wales migrants 1991 to 2001 stated that, although immigrants had an overall incidence of cancer commensurate with their proportional representation in the NSW population (24.5%), their rate of primary liver cancer was substantially higher, with 46% of all diagnoses occurring in overseas-born people.3 Standardised incidence ratios for primary liver cancer in men born in Vietnam, Hong Kong and Macau, Korea, Indonesia and China, and in women born in Vietnam and China, were at least six times those in Australian-born people3 (Box 1). In NSW, liver cancer shows geographic clustering, with rates in western Sydney far exceeding the NSW average (12.1 versus 4.8 cases per 100 000 population).4
Over 80% of primary liver cancer worldwide is attributable to the effects of chronic infection with hepatitis B or C virus (HBV or HCV).5 People with chronic HBV or HCV infection have a 20-fold to 200-fold greater risk than those not infected of developing hepatocellular carcinoma (HCC)6,7 (the most common form of primary liver cancer).
The high incidence of primary liver cancer in East Asia and South-East Asia is largely attributable to the high prevalence of chronic HBV infection in these regions.8 The early age of infection of people in these regions accounts for significant differences in the clinical course of the disease (with the prolonged immune-tolerance phase setting the scene for development of long-term complications) compared with people born in countries where hepatitis B prevalence is low (who generally become infected as teenagers or adults and enter the immune-clearance phase soon after developing chronic HBV infection). This also explains the former group’s worse prognosis and response to treatment.9 Screening for hepatitis B has been advocated for at-risk populations born in countries with a high prevalence of HBV infection, although the management pathways for individuals with chronic infection detected by screening programs remain ill defined.
Between 15% and 40% of people with chronic HBV infection develop liver-related morbidity, including cirrhosis, liver failure and liver cancer.9,10 The annual rate of progression to cirrhosis in people with chronic HBV infection ranges from 2%–5% in those who are positive for hepatitis B e antigen (HBeAg) to 8%–10% in those who are HBeAg-negative.9 Most, but not all, liver cancers occur in cirrhotic livers; in people with cirrhosis, the annual incidence of primary liver cancer ranges from 2%–3% in Western countries to 6%–11% in Asian populations.11
The recent demonstration of a close correlation between HBV replication and the risk of disease progression and liver cancer,12-14 coupled with data suggesting that effective suppression of viral replication may reduce the risk of primary liver cancer,12-15 represents a paradigm shift in cancer prevention strategies. However, this raises more questions, including the optimal time to initiate treatment, so as to reduce the incidence of resistance to inhibitors of viral replication, manage the adverse effects of prolonged antiviral therapy and ensure treatment compliance.
It also raises the question of how to identify those at risk of HCC. A screening strategy for liver cancer that takes into account the level of viral load has been suggested by the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-HBV (REVEAL) study, which showed that an elevated serum HBV DNA level (> 10 000 copies/mL) is a strong predictor of liver cancer risk, independent of any other markers of activity, such as HBeAg, alanine aminotransferase and cirrhosis.16
A randomised controlled trial that enrolled over 18 000 people with chronic hepatitis B showed a 37% reduction in mortality among those offered liver cancer screening compared with those allocated to usual medical care.17 However, many questions remain about the optimal use of cancer screening, including the cost-effectiveness of screening protocols and the potential of antiviral treatment to prevent primary liver cancer. The low cure rate for patients diagnosed from symptoms (5-year survival rates below 10%) and the significant differences in size and number of screen-detected compared with clinically detected cancers11 drive the use of cancer screening as a means of increasing the proportion of cancers amenable to curative interventions.
Informal liver cancer screening is already common practice in the developed world, as evidenced by a survey of United States gastroenterologists, 84% of whom screened their patients with cirrhosis for liver cancer.18 It has been proposed to focus surveillance for liver cancer on groups with a higher baseline risk, such as people with chronic hepatitis B or C born in Asian or African countries, and people with a family history of primary liver cancer,19 but the tests or combination of tests to use, and the optimal screening intervals and follow-up strategies remain uncertain. Therapeutic options for primary liver cancer shown to be associated with survival benefit include surgical resection, liver transplantation, percutaneous injection and radiofrequency ablation.20
In Australia, estimates of the numbers of people with chronic hepatitis B range from 90 000 to 160 000.21 Most carriers are born in countries with a high prevalence of chronic HBV infection. Although people born in China and Vietnam represent only about 5% of the total population, they make up about half of all cases of chronic hepatitis B.22 A hospital-based series found that people born in Asia, the Pacific Islands, North Africa, the Middle East or Mediterranean countries are at least six times more likely to be diagnosed with chronic hepatitis B than people born in Australia.23 The report on cancer incidence in NSW immigrants stated that people born in these regions were two to 12 times more likely to be diagnosed with primary liver cancer than people born in Australia (Box 1),3 underscoring the need for a public health approach to reducing cancer-related morbidity and mortality in at-risk populations.
A retrospective analysis of national incidence and mortality data for liver cancer from 1983 to 1996 was published in the Journal in 2000, and reported substantial increases in the incidence and liver cancer-related death rates in Australia in all population groups (except Australian-born women), postulating it to be related to the rising prevalence of chronic HBV and HCV infections.24 An accompanying editorial stated that “we are in a unique position to introduce initiatives that may influence this disturbing trend”.25
The year 2000, when this analysis and editorial were published,24,25 was the first year of the National Hepatitis C Strategy. This put forward a broad agenda for disease control, including building partnerships, involving affected communities, preventing HCV transmission, providing clinical treatment and other care and support for people affected by hepatitis C, training and education, and supporting research and surveillance activities.26 Eight years later, the initial strategy has been transformed, with more committed primary prevention strategies, improved access to and removal of barriers to treatment, more involvement of the primary health care sector in the diagnosis and management of HCV infection, and a more supportive environment for people affected by this condition.
Although population groups at highest risk of chronic infection are well defined, screening for chronic HBV infection as a pathway to evaluation and appropriate management is not yet on the Australian public health agenda. This contrasts with the public health response in New Zealand, where the Hepatitis B Screening Programme has screened more than 177 000 people in high-risk groups for evidence of HBV infection, and identified more than 12 000 people with chronic hepatitis B and enrolled them in a primary liver cancer surveillance program.27 A similar initiative was coordinated by the Asian American Hepatitis B Program in New York, aiming to identify people with chronic HBV infection among Asian and Pacific Islander communities, to provide a pathway for preventing the complications of chronic liver disease in those infected, and to offer HBV vaccination to susceptible contacts.28 Many Asian countries have also adopted screening programs for patients at risk,8 and Taiwan has implemented a community-based HCC screening program, which also offers screening for family members of liver cancer patients.29
It is likely that the incidence of liver cancer in Australia will continue to rise for at least the next two decades, as a result of the combined effect of high levels of immigration from countries where HBV infection is endemic and the relatively slow rates of disease progression.22
Although we are not yet winning the war on liver cancer, we can develop successful strategies for addressing this challenge. We can capitalise on the pioneering initiative in New Zealand and the local experience gained through developing the public health response to hepatitis C. A broad engagement with key stakeholders, coupled with government commitment, would ensure that the actions summarised in Box 2 can be effectively implemented as workable solutions to this significant public health challenge. Without such commitment, broader questions about treatment and prevention will remain subjects of debate rather than shape best practice.
1 Standardised incidence ratios* for primary liver cancer in New South Wales immigrants, by place of birth (1991–2001)3
 | |||||||||||||||
2 Elements of a public health response to hepatitis B and liver cancer
Raising liver cancer awareness in at-risk communities and among health practitioners
Developing programs encouraging people at risk to know their hepatitis B or hepatitis C virus status
Promoting informed decisions about testing, surveillance and management for people with chronic hepatitis B
Encouraging timely and appropriate referral of patients with chronic hepatitis B for assessment and possible antiviral treatment
Stimulating public health and clinical research on hepatitis B virus and liver cancer
Mobilising public resources to effectively meet the costs of screening and treatment pathways
Ensuring that community advocacy informs hepatitis B virus policy and practice
Developing the skills of health practitioners working with affected communities, at all levels.
- Monica C Robotin1,2
- Jacob George3
- Rajah Supramaniam1
- Freddy Sitas1
- Andrew G Penman1
- 1 The Cancer Council NSW, Sydney, NSW.
- 2 School of Public Health, University of Sydney, Sydney, NSW.
- 3 Department of Gastroenterology and Hepatology, Storr Liver Unit, Westmead Millenium Institute, University of Sydney and Westmead Hospital, Sydney, NSW.
None identified.
- 1. Australian Institute of Health and Welfare, Australasian Association of Cancer Registries. Cancer in Australia 2001. Canberra: AIHW, 2004. (AIHW Cat. No. CAN 23.) http://www.aihw.gov.au/publications/can/ca01/ca01.pdf (accessed Oct 2007).
- 2. Coates M, Tracey E. Cancer in New South Wales: incidence and mortality 1997. N S W Public Health Bull 2001; 12: 40-42.
- 3. Supramaniam R, O’Connell DL, Tracey E, Sitas F. Cancer incidence in New South Wales migrants 1991 to 2001. Sydney: The Cancer Council NSW, 2006. http://www.cancercouncil.com.au/editorial.asp?pageid=2253 (accessed Oct 2007).
- 4. Tracey E, Chen S, Baker D, et al. Cancer in New South Wales: incidence and mortality 2004. Sydney: Cancer Institute NSW, 2006. http://www.cancerinstitute.org.au/cancer_inst/publications/pdfs/IncidenceMortality Report2004.pdf (accessed Oct 2007).
- 5. Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118: 3030-3044.
- 6. Amin J, Dore GJ, O’Connell DL, et al. Cancer incidence in people with hepatitis B or C infection: a large community-based linkage study. J Hepatol 2006; 45: 197-203.
- 7. Chu CM. Natural history of chronic hepatitis B virus infection in adults with emphasis on the occurrence of cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol 2000; 15 Suppl: E25-E30.
- 8. Teo EK, Fock KM. Hepatocellular carcinoma: an Asian perspective. Dig Dis 2001; 19: 263-268.
- 9. Yuen MF. Revisiting the natural history of chronic hepatitis B: impact of new concepts on clinical management. J Gastroenterol Hepatol 2007; 22: 973-976.
- 10. Schiff ER. Prevention of mortality from hepatitis B and hepatitis C. Lancet 2006; 368: 896-897.
- 11. De Masi S, Tosti ME, Mele A. Screening for hepatocellular carcinoma. Dig Liver Dis 2005; 37: 260-268.
- 12. Fung SK, Lok AS. Treatment of chronic hepatitis B: who to treat, what to use, and for how long? Clin Gastroenterol Hepatol 2004; 2: 839-848.
- 13. Lai CJ, Terrault NA. Antiviral therapy in patients with chronic hepatitis B and cirrhosis. Gastroenterol Clin North Am 2004; 33: 629-654.
- 14. Lok AS, McMahon BJ. Chronic hepatitis B: update of recommendations. Hepatology 2004; 39: 857-861.
- 15. Hache C, Villeneuve JP. Lamivudine treatment in patients with chronic hepatitis B and cirrhosis. Expert Opin Pharmacother 2006; 7: 1835-1843.
- 16. Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295: 65-73.
- 17. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130: 417-422.
- 18. Chalasani N, Said A, Ness R, et al. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol 1999; 94: 2224-2229.
- 19. Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis 2005; 25: 143-154.
- 20. Perry JF, Poustchi H, George J, et al. Current approaches to the diagnosis and management of hepatocellular carcinoma. Clin Exp Med 2005; 5: 1-13.
- 21. O’Sullivan BG, Gidding HF, Law M, et al. Estimates of chronic hepatitis B virus infection in Australia, 2000. Aust N Z J Public Health 2004; 28: 212-216.
- 22. Dore G, Wallace J, Locarnini S, et al. Hepatitis B in Australia: responding to a diverse epidemic. Sydney: Australasian Society for HIV Medicine, 2006. http://www.ashm.org.au/uploads/Hep-B-in-Australia.pdf (accessed Oct 2007).
- 23. Tawk HM, Vickery K, Bisset L, et al. The current pattern of hepatitis B virus infection in Australia. J Viral Hepat 2006; 13: 206-215.
- 24. Law MG, Roberts SK, Dore GJ, Kaldor JM. Primary hepatocellular carcinoma in Australia, 1978-1997: increasing incidence and mortality. Med J Aust 2000; 173: 403-405.
- 25. Crawford DH, Fawcett J. Hepatocellular carcinoma in Australia: largely preventable [editorial]? Med J Aust 2000; 173: 396-397.
- 26. Commonwealth Department of Health and Aged Care. National Hepatitis C Strategy 1999–2000 to 2003–2004. Canberra: Commonwealth of Australia, 2000.
- 27. Robinson T, Bullen C, Humphries W, et al. The New Zealand Hepatitis B Screening Programme: screening coverage and prevalence of chronic hepatitis B infection. N Z Med J 2005; 118: U1345.
- 28. Centers for Disease Control and Prevention. Screening for chronic hepatitis B among Asian/Pacific Islander populations — New York City, 2005. MMWR Morb Mortal Wkly Rep 2006; 55: 505-509.
- 29. Cancer Expert Working Group on Cancer Prevention and Screening. Report of the Cancer Expert Working Group on Cancer Prevention and Screening. Hong Kong: Department of Health, 2004.





Abstract
Worldwide, over 80% of primary liver cancers are attributable to chronic infection with hepatitis B or C virus.
Over the past two decades, primary liver cancer incidence rates have been consistently rising in Australia.
In New South Wales, the standardised incidence ratios for primary liver cancer in males born in Vietnam, Hong Kong and Macau, Korea, Indonesia and China and in females born in Vietnam and China are 6–12 times those in Australian-born populations.
The incidence of liver cancer is likely to continue to increase unless a coordinated approach to disease control can be developed.
Effective programs for chronic hepatitis B management need to link prevention, treatment and care, and enhance opportunities for research and surveillance activities.
The evidence that suppression of hepatitis B virus replication could limit disease progression needs to inform the development of a public health response.
Lessons learned in the development of the National Hepatitis C Strategy and the experience of international hepatitis B control programs need to inform this process.