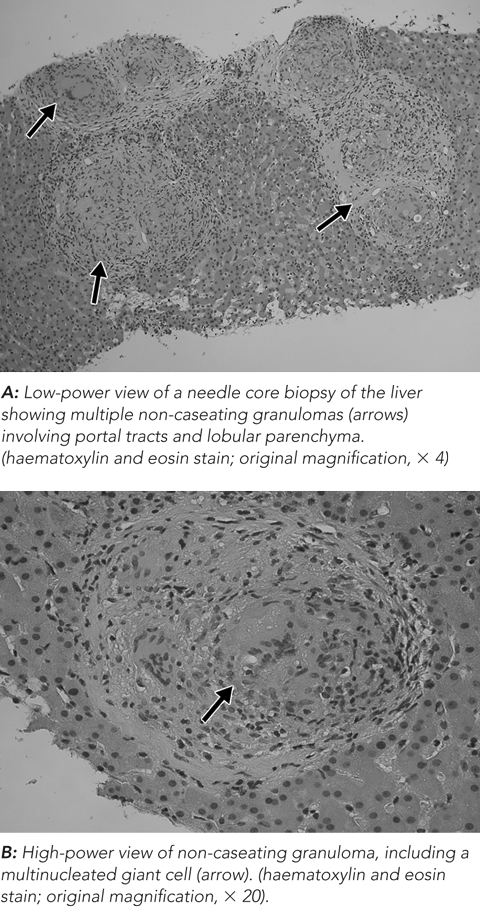
Granulomatous hepatitis is an uncommon condition with a lengthy list of possible causes,1 as shown in Box 1. In our patient, the biopsy did not show bile duct destruction characteristic of primary biliary cirrhosis, or any evidence of malignancy. The patient was not taking any drugs which could cause granulomatous hepatitis. The pathology laboratory report suggested the diagnosis was sarcoidosis, and, of the infections that can cause granulomatous hepatitis, tuberculosis (TB) is the most common. Hence, although the differential diagnosis is long, the main clinical decision related to the ability to diagnose or exclude TB. If a patient with hepatic TB has corticosteroid therapy for erroneously diagnosed sarcoidosis, the consequences may be catastrophic. Active TB most typically presents with pulmonary symptoms, and the diagnosis can usually be made provided smears and cultures for mycobacteria are obtained. However, if a patient presents more atypically, as did our patient, clinicians may initially not suspect TB as the diagnosis.2
Lessons from practice
It is critical to be certain that the cause of granulomatous hepatitis is not tuberculosis (TB) before commencing immunosuppressive medication.
TB remains easy to diagnose if mycobacteria can be detected, but active TB is very difficult to exclude if cultures are negative or not performed.
Risk of TB is determined by patient’s country of birth and duration of time spent there before migration.13
If TB cannot be excluded, a trial of antituberculosis therapy may be warranted.
The TST is often falsely negative in immunosuppressed patients, and can give false-positive results if the patient has had previous BCG vaccination3 or infection with non-tuberculous types of mycobacteria. QuantiFERON-TB Gold is more specific for TB,4 but its sensitivity is poor, especially with immunosuppression.5 Both of these tests can be falsely negative in up to 20% of patients with active TB, and neither the TST nor the QuantiFERON-TB Gold test can distinguish between current, latent and previous (treated) infection.
The only way to definitively diagnose active TB infection is by culturing the organism, or by detecting its nucleic acid sequence by PCR amplification from tissue or fluid samples. If active disease is suspected, specimens should be cultured for M. tuberculosis whenever possible, as a positive result confirms the diagnosis and allows drug sensitivity tests to be performed. While the diagnosis of active TB can be confirmed by PCR, this is not necessarily as sensitive as culture, and does not allow drug sensitivity testing.6
In our patient, the mycobacterium was confined to the liver, which was unusual. It is far more common to have TB involvement of the liver in disseminated TB.7 Ideally, the liver biopsy should have been sent for culture in a TB medium, but we did not suspect TB initially. Once fixed in formalin and paraffin, the tissue cannot be cultured, but PCR of M. tuberculosis DNA can still be performed on the specimen and may yield a diagnosis.8
Histological examination of liver tissue provides crucial information in the differential diagnosis of hepatic granulomas. While caseous necrosis is characteristic of TB infection, it is not always present and its absence cannot be used to exclude TB.7 Also, as it is often difficult to detect the presence of acid-fast bacilli (AFB) within the granulomas, the absence of AFB cannot be used to exclude TB either.9
In Australia, the incidence of TB is six per 100 000 per year,10 compared with the incidence of sarcoidosis, which is estimated at 20 per 100 000 per year worldwide;11 sarcoidosis is thus the more likely cause of hepatic granulomas in Australian-born patients. However, the incidence of TB in the Philippines is 291 per 100 000 per year,12 making TB the far more likely cause of hepatic granulomas in our Filipino-born patient.
1 Causes of granulomatous hepatitis
Autoimmune
Sarcoidosis
Primary biliary cirrhosis
Systemic infections
Mycobacterial — tuberculosis
Fungal — cryptococcosis
Rickettsial — Q fever
Zoonotic — brucellosis
Malignancy
Hodgkin’s disease
Non-Hodgkin’s lymphoma
Renal cell carcinoma
Drugs
Allopurinol
Sulphur drugs
Quinidine
Other drugs
Idiopathic
- 1. Zakim D, Boyer TD, editors. Hepatology: a textbook of liver disease. 3rd ed. Vol 3. Philadelphia: WB Saunders, 1996: 1472.
- 2. Klatskin G. Hepatic granulomata: problems in interpretation. Mt Sinai J Med 1977; 44: 798.
- 3. Tissot F, Zanetti G, Francioli P, et al. Influence of bacilli Calmette–Guerin vaccination on size of tuberculin skin reaction: to what size? Clin Infect Dis 2005; 40: 211-217.
- 4. Pai M, Riley LW, Colford JM Jr. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 2004; 4: 761.
- 5. Liebeschuetz S, Bamber S, Ewer K, et al. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet 2004; 364: 2196-2203.
- 6. Wiener RS, Della-Latta P, Schluger NW. Effect of nucleic acid amplification for Mycobacterium tuberculosis on clinical decision making in suspected extrapulmonary tuberculosis. Chest 2005; 128: 102.
- 7. Alvarez SZ, Carpio R. Hepatobiliary tuberculosis. Dig Dis Sci 1983; 28: 193.
- 8. Akcan Y, Tuncer S, Hayran M, et al. PCR on disseminated tuberculosis in bone marrow and liver biopsy specimens: correlation to histopathological and clinical diagnosis. Scand J Infect Dis 1997; 29: 271.
- 9. Alcantara-Payawal DE, Matsumura M, Shiratori Y, et al. Direct detection of Mycobacterium tuberculosis using polymerase chain reaction assay among patients with hepatic granuloma. J Hepatol 1997; 27: 620-627.
- 10. World Health Organization. TB country profile. Australia. Geneva: WHO, 2005. http://www.who.int/GlobalAtlas/predefinedReports/TB/PDF_Files/aus.pdf (accessed Nov 2007).
- 11. Thomas KW, Hunninghake GW. Sarcoidosis. JAMA 2003; 289: 3300.
- 12. World Health Organization. Country profile. Philippines. Geneva: WHO, 2005. http://www.who.int/GlobalAtlas/predefinedReports/TB/PDF_Files/phl.pdf (accessed Nov 2007).
- 13. Johnson PDR, Carlin JB, Bennett CM, et al. Prevalence of tuberculosis infection in Melbourne secondary school students. Med J Aust 1998; 168: 106-110.





None identified.