Australia is fortunate to have one of the lowest incident rates of tuberculosis (TB) in the world, with rates remaining stable at 5–6 cases per 100 000 population since the mid 1980s.1 However, Australia’s close proximity to many countries in the Asia–Pacific region with a high or intermediate TB burden necessitates an evaluation of policies and strategies for TB control for the Australian population.
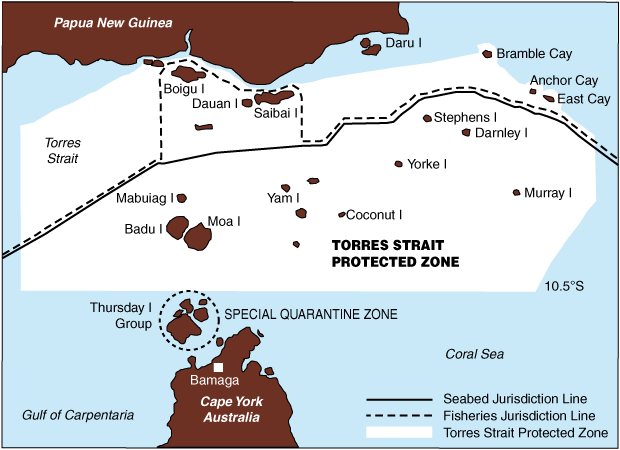
Just north of the Australian mainland lies the Torres Strait, which separates Australia from Papua New Guinea (PNG). The health status of Papua New Guineans is one of the lowest in the Pacific region, and TB is one of the three leading infectious diseases causing death in PNG, with an estimated mortality rate of 42 deaths per 100 000 population. The estimated annual incidence of all forms of TB in PNG is 233 cases per 100 000 population.2
A treaty between PNG and Australia to establish a maritime boundary between the two countries was ratified in 1985. The Seabed Jurisdiction Line effectively marks the boundary. Australia has sovereignty below the line and PNG has rights north of the line, although PNG recognises Australia’s sovereignty over 15 islands and cays north of the line, including Boigu, Saibai and Dauan (Box 1). These three islands are very close to the mainland of the Western Province of PNG. The treaty also established a “Torres Strait Protected Zone”, whereby Australians who are Torres Strait Islanders and Papua New Guineans who live in the coastal areas of PNG are permitted to migrate across the borders in accordance with the livelihood and way of life of the traditional inhabitants. This presents an important challenge to Australia to ensure that the unique lifestyle of the region’s traditional inhabitants is protected while advancing Australia’s public health interests. Because of limited access to health care in the Western Province of PNG, PNG nationals from the coastal areas are travelling to the nearby islands of Boigu and Saibai to seek treatment.
Genotyping was performed on each strain using both variable number tandem DNA repeat (VNTR) typing3 and 12-locus mycobacterial interspersed repetitive unit (MIRU) typing.4 VNTR typing used three exact tandem repeat loci (A, B and C) plus two of the MIRU loci (MIRU 4 and MIRU 31) corresponding to exact tandem repeat loci D and E to generate a five-digit profile. VNTR and MIRU loci were amplified by the polymerase chain reaction (PCR), using specific primers complementary to the flanking regions. The number of tandem repeats at each locus was determined by estimating the size of PCR products on agarose gels. The results were expressed as numerical profiles in which each digit represented the number of copies at a particular locus.
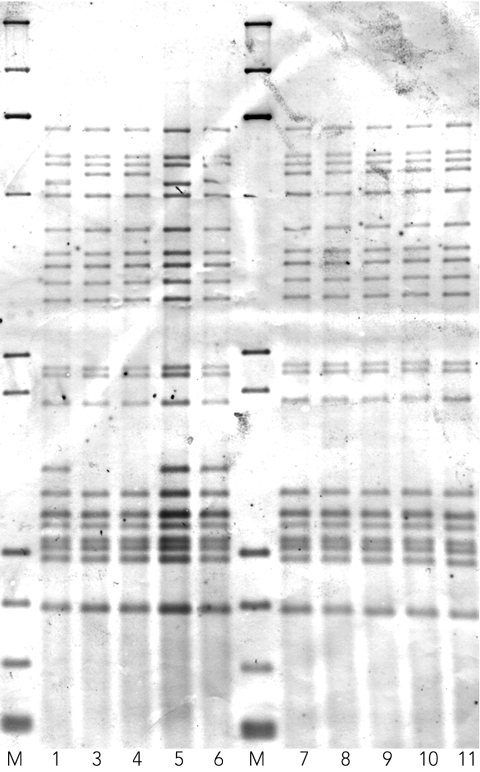
Ten of the 15 MDR-TB strains were available to confirm clustering by insertion sequence 6110 (IS6110) restriction fragment length polymorphism (RFLP) typing.5 In our study, an IS6110-RFLP cluster was defined as a collection of isolates that shared 100% fingerprint identity.
Between 2000 and 2006, there were 60 cases of M. tuberculosis identified among Papua New Guineans presenting to clinics in the region that were bacteriologically confirmed by the Mycobacterium Reference Laboratory in Brisbane. Of the TB strains isolated, 15 (25%) were MDR-TB strains resistant to at least isoniazid and rifampicin. No extensively drug-resistant strains (ie, strains additionally resistant to fluoroquinolones and at least one of the three second-line injectable drugs [amikacin, kanamycin and capreomycin]) were detected. Drug susceptibility and genotyping results for the 15 MDR-TB strains, together with patient outcomes and treatment regimens, are summarised in Box 2.
The IS6110-RFLP patterns for 10 of the 15 strains isolated between 2000 and 2006 are shown in Box 3. Two distinct fingerprints were evident. Cluster A comprised three patients (1, 5 and 6) with an identical 21-copy IS6110 pattern. These three subjects all originated from the coastal village of Mabadauan in the Western Province of PNG, which is opposite Saibai Island (one of the Torres Strait Islands under Australia’s jurisdiction), where the patients were diagnosed. Cluster B comprised seven patients (3, 4 and 7–11) with an identical 20-copy IS6110 pattern. These patients were diagnosed at Boigu Island, another Torres Strait Island of Australia. The patients in the larger cluster originate from a number of villages in the Western Province of PNG. Sigabaduru village is on the PNG mainland across from Saibai Island, with Mabadauan slightly further away. Buzi, a coastal village opposite Boigu Island, is further west on the PNG mainland towards the West Papuan border. Dimiri, a small PNG village inland from Buzi, is not within the Torres Strait Protected Zone, so people from this village do not have traditional visitation rights into the Torres Strait Islands. Daru Island (under PNG’s jurisdiction) is east of Mabadauan and Sigabaduru and contains the major hospital for patients within the South Fly Health Service District of the Western Province. This hospital is the official PNG referral hospital for the PNG villages contained within the Torres Strait Protected Zone. Thus the seven-patient cluster came from a wide geographical area.
Patient outcome details are summarised in Box 2. Of the 15 patients with MDR-TB, six died, a further two were lost to follow-up (and are presumed to have died), three are currently being treated and showing signs of clinical improvement, and four (patients 7, 9, 10 and 11) have completed treatment, with apparent clinical and radiological cure. (Bacteriological quiescence has been proven in patient 10, but the other three have been unable to produce satisfactory sputum specimens.)
The occurrence of MDR-TB strains adds to the complexity of TB control in the Torres Strait region. Our genotyping results highlight the potentially serious problem of primary transmission of MDR-TB in the Western Province of PNG. MDR-TB poses a serious threat to infected individuals and to whole communities.6
Both clusters of MDR-TB strains detected were Beijing-family strains. The Beijing family is most prevalent in South-East Asian countries and is increasingly found to be associated with MDR-TB.7,8 These strains have also been associated with increased transmissibility in communities,9,10 which may be associated with increased virulence, although this has not been proven.11
Although clear hotspots for MDR-TB have been identified in eastern Europe and parts of China, it appears that MDR-TB is limited to local epidemics.11 Adoption of directly observed therapy short course (DOTS) to prevent the generation of resistant strains and careful introduction of second-line drugs to treat patients with MDR-TB are the top priorities for proper containment of MDR-TB.12 However, DOTS programs are poorly implemented in PNG, and there are inadequate resources to determine the extent of transmission of MDR-TB.13
In early 2006, Simpson and colleagues reported a rising incidence of TB in the Torres Strait and changing TB patterns in Far North Queensland.12 Evidence of rising incidence of TB in the Torres Strait and primary transmission of MDR-TB within the Western Province of PNG suggests the potential for a major public health crisis, with the possibility of MDR-TB spreading to northern Queensland. Urgent efforts are needed to scale up resources to find further cases and provide appropriate treatment and follow-up for all visitors and residents living in the Torres Strait Protected Zone. The failure to contain an epidemic of MDR-TB now may have significant long-term financial and social implications for both PNG and Australia.
1 Map of the Torres Strait region, showing the boundaries between Australian and Papua New Guinean territory
2 Genotyping,* drug susceptibility testing results, treatment regimens and patient outcomes for the 15 MDR-TB isolates
- Christopher M Gilpin1
- Graham Simpson2
- Stephen Vincent2
- Terry P O’Brien3
- Trevor A Knight2
- Maria Globan4
- Christopher Coulter1
- Anastasios Konstantinos3
- 1 Mycobacterium Reference Laboratory, Pathology Queensland, Brisbane, QLD.
- 2 Cairns Regional TB Control Unit, Cairns Base Hospital, Cairns, QLD.
- 3 Queensland TB Control Centre, Queensland Health, Brisbane, QLD.
- 4 Mycobacterium Reference Laboratory, Victorian Infectious Diseases Reference Laboratory, Melbourne, VIC.
We would like to thank Dr Suparat Phuanukoonon of the PNG Institute of Medical Research, Goroka, and Dr James McCarthy of the Queensland Institute of Medical Research for sharing unpublished drug sensitivity data from clinical isolates collected in Goroka and Madang.
None identified.
- 1. Roche P, Bastian I, Krause V, et al. Tuberculosis notifications in Australia, 2005. Commun Dis Intell 2007; 31: 71-80.
- 2. World Health Organization. WHO Report 2006. Global tuberculosis control: surveillance, planning, financing. Geneva: WHO, 2006. (WHO/HTM/TB/2006.362.)
- 3. Frothingham R, Meeker-O’Connell WA. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology 1998; 144: 1189-1196.
- 4. Cowan LS, Mosher L, Diem L, et al. Variable-number tandem repeat typing of Mycobacterium tuberculosis isolates with low copy numbers of IS6110 by using mycobacterial interspersed repetitive units. J Clin Microbiol 2002; 40: 1592-1602.
- 5. van Embden JD, Cave MD, Crawford JT, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 1993; 31: 406-409.
- 6. Narvskaya O, Otten T, Limeschenko E, et al. Nosocomial outbreak of multidrug-resistant tuberculosis caused by a strain of Mycobacterium tuberculosis W-Beijing family in St Petersburg, Russia. Eur J Clin Microbiol Infect Dis 2002; 21: 596-602.
- 7. Mokrousov I, Otten T, Vyazovaya A, et al. PCR-based methodology for detecting multidrug-resistant strains of Mycobacterium tuberculosis Beijing family circulating in Russia. Eur J Clin Microbiol Infect Dis 2003; 22: 342-348.
- 8. Munckhof WJ, Konstantinos A, Wamsley M, et al. A cluster of tuberculosis associated with use of a marijuana water pipe. Int J Tuberc Lung Dis 2003; 7: 860-865.
- 9. Drobniewski F, Balabanova Y, Ruddy M, et al. Rifampin- and multidrug-resistant tuberculosis in Russian civilians and prison inmates: dominance of the beijing strain family. Emerg Infect Dis 2002; 8: 1320-1326.
- 10. Park YK, Shin S, Ryu S, et al. Comparison of drug resistant genotypes between Beijing and non-Beijing family strains of Mycobacterium tuberculosis in Korea. J Microbiol Methods 2005; 63: 165-172.
- 11. Espinal MA. The global situation of MDR-TB. Tuberculosis (Edinb) 2003; 83: 44-51.
- 12. Simpson G, Clark P, Knight T. Changing patterns of tuberculosis in Far North Queensland [letter]. Med J Aust 2006; 184: 252. <MJA full text>
- 13. Levy MH, Dakulala P, Koiri JB, et al. Tuberculosis control in Papua New Guinea. P N G Med J 1998; 41: 72-76.





Abstract
Objective: To review patient outcomes and the molecular epidemiology of multidrug-resistant tuberculosis (MDR-TB) strains isolated from patients living in the Western Province of Papua New Guinea (PNG) seeking treatment in Australia.
Design, setting and participants: Review of all cases of MDR-TB among people living in the open border region between the Western Province of PNG and the Torres Strait Islands of Australia who presented to health clinics in the region between 2000 and 2006. All cases of suspected TB were bacteriologically confirmed at the time of presentation by the Mycobacterium Reference Laboratory in Brisbane.
Main outcome measures: Drug resistance patterns; drug use and duration; molecular typing of TB strains; patient outcomes.
Results: Between 2000 and 2006, 60 patients from the Western Province of PNG were diagnosed with TB, of which 15 had MDR-TB. Mortality was high, although no patient who was able to maintain access to supervised therapy died. All 15 MDR-TB isolates were Beijing-family strains showing the same unique mycobacterial interspersed repetitive unit (MIRU) profile, with the exception of a single strain that differed by a single repeat at one locus. Restriction fragment length polymorphism (RFLP) typing on 10 of these strains further differentiated them into two distinct clusters.
Conclusion: Transmission of MDR-TB is occurring in the Western Province of PNG. Additional resources are urgently needed to interrupt the ongoing transmission of MDR-TB from the Western Province of PNG to the Torres Strait Islands. Good supervision and management of patient treatment, which includes ensuring a regular supply of second-line anti-TB drugs, are essential elements of TB control.