Conventional treatment of saphenous vein reflux by surgical ligation or stripping leads to appreciable trauma, disruption of activities, scarring and high late recurrence rates.1 Alternative, non-surgical techniques are gaining increasing acceptance. Endovenous laser therapy (EVLT) provides a percutaneous technique to destroy larger diameter saphenous veins as an outpatient procedure under local anaesthesia, with minimal disruption of activities and no surgical trauma.2-5 In this article, we describe our early results for 404 saphenous veins in 308 patients treated by EVLT and followed up by ultrasound surveillance.
The clinical, aetiological, anatomical, and pathophysiological (CEAP) classification was used to assess the limbs.6 There were 361 limbs with uncomplicated varicose veins (C2–3, 91%) and 35 limbs with complications (C4–6), due to lipodermatosclerosis (n = 26), healed past venous ulceration (n = 6) or active ulceration (n = 3). Primary disease was present in all limbs, and none had features of the post-thrombotic syndrome. There was persistent or recurrent reflux after past saphenous vein surgery by other surgeons in 20 limbs (15 for great saphenous and 5 for small saphenous disease).
Duplex scanning performed by specialist vascular sonographers linked to the surgical units was used to select saphenous veins suitable for EVLT. Limbs were evaluated to detect superficial, deep and perforator reflux, mark the site and extent of disease, and measure the length and diameters of refluxing saphenous veins as previously described.7 All limbs treated had reflux through the corresponding saphenous junction or other major connections to deep veins. The lengths of veins treated ranged from 5 to 55 cm (median, 34 cm) and their diameters from 5 to 20 mm (median, 8 mm).
Follow-up with serial ultrasound scans at the above intervals was used for survival analysis.8 Success was defined as continuing occlusion or obliteration without reflux in any segment of treated vein, as determined by ultrasound.
Univariate Kaplan–Meier life table analysis was used to calculate primary and secondary ultrasound success and failure rates.8 The time to failure was the difference between the date of EVLT and the date that recurrent reflux was demonstrated at follow-up scans. All patients presented for the first post-procedure scan at day 3–7; if failure was noted at this scan, then this was used as the failure date for survival analysis, although it is probable that the procedure had failed from the time it was performed. If a patient noted to have recurrent saphenous reflux had missed a previous scheduled visit, then failure due to recurrent saphenous reflux was dated back to the time of that missed visit.
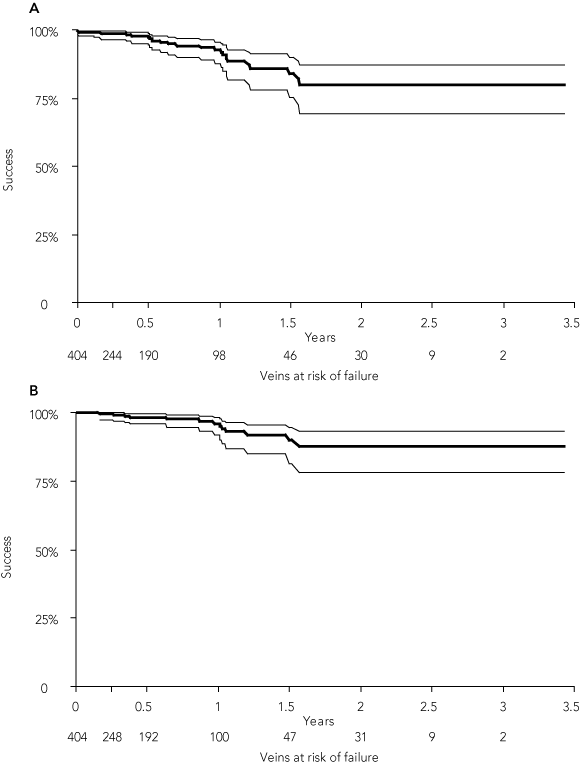
In 21 limbs, recanalisation was detected on surveillance, usually to a minor degree when compared with the initial reflux. This resulted in a primary ultrasound success rate at 3 years by life table analysis of 80% (95% CI, 69%–87%) (Box 1A). Eleven of these limbs were treated by ultrasound-guided sclerotherapy to obliterate the recurrent vein at intervals from 7 to 570 days after EVLT, and this was successful in all but one, resulting in a secondary ultrasound success rate at 3 years by life table analysis of 88% (95%,CI, 78%–95%) (Box 1B).
Thromboembolic complications can occur with any treatment for varicose veins. In this series, EVLT had a 2.2% incidence of thromboembolic complications. Van Rij and colleagues documented deep vein thrombosis in 5.3% of limbs after varicose vein surgery, although most were localised to the tibial veins.9
EVLT damages a blood-filled vessel by steam formation, leading to endothelial denudation, collagen contraction and vein wall fibrosis,10-13 and in many limbs the vein is no longer visible at the 6–12 month scans. Our results are similar to those in another large series.2 Other studies have reported satisfactory results for the great saphenous3,4 and small saphenous veins.5
An alternative technique using thermal ablation from a radiofrequency probe has also produced good results with low complication rates.14-17 Ultrasound surveillance shows occlusion of most saphenous veins and infrequent development of new veins in the groin with this technique.16 Randomised trials of radiofrequency closure versus surgery found significantly less postoperative pain, faster rehabilitation, lower cost and persisting better quality of life, as well as similar control of the veins.14-17
Outcomes are satisfactory for treatment of saphenous reflux by ultrasound-guided sclerotherapy,18-20 but there is insufficient information to determine the efficacy of ultrasound-guided sclerotherapy for larger saphenous veins.
Ultrasound surveillance detects a high incidence of failure after surgery for varicose veins.1 Van Rij and colleagues found 25% recurrence after great saphenous surgery and 50% recurrence after small saphenous surgery at 3 years.21 A Swedish study of outcome 10 years after great saphenous ligation and stripping found that 86 of 100 limbs had recurrence involving segments of the great saphenous veins.22 Ultrasound studies after small saphenous surgery found that only 39% of 59 operations were successful at early follow-up in a British report,23 and 5 of 28 operations were successful at 3 months in a Dutch study.24 A British review suggests that this may be due to reluctance to strip the small saphenous vein because of fear of nerve injury.25
There is a high incidence of reconnection from the common femoral vein or low abdominal or pelvic veins to thigh tributaries after surgery, due to opening of pre-existing veins.26,27 Traditional teaching is to ligate all tributaries at the saphenofemoral junction, but there is growing concern that this might predispose to reconnections into thigh veins rather than normal drainage through the saphenous junction. Endovenous techniques are not associated with a high incidence of recurrence in the groin,28 suggesting that leaving tributaries from above the groin may be an advantage.
Received 18 April 2005, accepted 20 May 2006
- Kenneth Myers1
- Robert Fris2
- Damien Jolley3
- 1 Epworth Hospital, Melbourne, VIC.
- 2 Northern Vein Centre, Auckland, New Zealand.
- 3 Monash Institute of Health Services Research, Melbourne, VIC.
We thank our sonographers and nursing sister — Amy Clough, Jacqui Kirwan, Michelle Rodeh, Jane Chambers and Penny Koh in Melbourne, and Bronwyn Allen and Daryl Queenin in Auckland — for their invaluable assistance and advice.
None identified.
- 1. Perrin MR, Guex JJ, Ruckley CV, et al. Recurrent varices after surgery (REVAS), a consensus document. REVAS group. Cardiovasc Surg 2000; 8: 233-245.
- 2. Min RJ, Khilnani N, Zimmet SE. Endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Interv Radiol 2003; 14: 991-996.
- 3. Proebstle TM, Gul D, Lehr HA, et al. Infrequent early recanalization of greater saphenous vein after endovenous laser treatment. J Vasc Surg 2003; 38: 511-516.
- 4. Timperman PE. Prospective evaluation of higher energy great saphenous vein endovenous laser treatment. J Vasc Interv Radiol 2005; 16: 791-794.
- 5. Proebstle TM, Gul D, Kargl A, Knop J. Endovenous laser treatment of the lesser saphenous vein with a 940-nm diode laser: early results. Dermatol Surg 2003; 29: 357-361.
- 6. Myers KA. Classification and grading of chronic venous disease in the lower limbs: a consensus statement. American Venous Forum. Aust N Z J Surg 1995; 65: 769-772.
- 7. Myers KA, Ziegenbein RW, Zeng GH, Matthews PG. Duplex ultrasonography scanning for chronic venous disease: patterns of venous reflux. J Vasc Surg 1995; 21: 605-612.
- 8. Lee ET. Statistical methods for survival data analysis. Belmont: Lifetime Learning Publications, 1980.
- 9. van Rij AM, Chai J, Hill GB, Christie RA. Incidence of deep vein thrombosis after varicose vein surgery. Br J Surg 2004; 91: 1582-1585.
- 10. Proebstle TM, Sandhofer M, Kargl A, et al. Thermal damage of the inner vein wall during endovenous laser treatment: key role of energy absorption by intravascular blood. Dermatol Surg 2002; 28: 596-600.
- 11. Proebstle TM, Lehr HA, Kargl A, et al. Endovenous treatment of the greater saphenous vein with a 940-nm diode laser: thrombotic occlusion after endoluminal thermal damage by laser-generated steam bubbles. J Vasc Surg 2002; 35: 729-736.
- 12. Min RJ, Khilnani NM. Endovenous laser treatment of saphenous vein reflux. Tech Vasc Interv Radiol 2003; 6: 125-131.
- 13. Proebstle TM, Krummenauer F, Gul D, Knop J. Nonocclusion and early reopening of the great saphenous vein after endovenous laser treatment is fluence dependent. Dermatol Surg 2004; 30: 174-178.
- 14. Rautio T, Ohinmaa A, Perala J, et al. Endovenous obliteration versus conventional stripping operation in the treatment of primary varicose veins: a randomized controlled trial with comparison of the costs. J Vasc Surg 2002; 35: 958-965.
- 15. Lurie F, Creton D, Eklof B, et al. Prospective randomized study of endovenous radiofrequency obliteration (closure procedure) versus ligation and stripping in a selected patient population (EVOLVeS Study). J Vasc Surg 2003; 38: 207-214.
- 16. Pichot O, Kabnick LS, Creton D, et al. Duplex ultrasound scan findings two years after great saphenous vein radiofrequency endovenous obliteration. J Vasc Surg 2004; 39: 189-195.
- 17. Lurie F, Creton D, Eklof B, et al. Prospective randomized study of endovenous radiofrequency obliteration (closure) versus ligation and vein stripping (EVOLVeS): two-year follow-up. Eur J Vasc Endovasc Surg 2005; 29: 67-73.
- 18. Kanter A, Thibault P. Saphenofemoral incompetence treated by ultrasound-guided sclerotherapy. Dermatol Surg 1996; 22: 648-652.
- 19. Belcaro G, Cesarone MR, Di Renzo A, et al. Foam-sclerotherapy, surgery, sclerotherapy, and combined treatment for varicose veins: a 10-year, prospective, randomized, controlled, trial (VEDICO trial). Angiology 2003; 54: 307-315.
- 20. Cabrera J, Cabrera J, Garcia-Olmedo MA. Treatment of varicose long saphenous veins with sclerosant in microfoam form: long-term outcomes. Phlebology 2000; 15: 19-23.
- 21. van Rij AM, Jiang P, Solomon C, et al. Recurrence after varicose vein surgery: a prospective long-term clinical study with duplex ultrasound scanning and air plethysmography. J Vasc Surg 2003; 38: 935-943.
- 22. Blomgren L, Johansson G, Dahlberg A, et al. Recurrent varicose veins: incidence, risk factors and groin anatomy. Eur J Vasc Endovasc Surg 2004; 27: 269-274.
- 23. Rashid HI, Ajeel A, Tyrrell MR. Persistent popliteal fossa reflux following saphenopopliteal disconnection. Br J Surg 2002; 89: 748-751.
- 24. Spronk S, Boelhouwer RU, Veen HF, den Hoed PT. Subfascial ligation of the incompetent short saphenous vein: technical success measured by duplex sonography. J Vasc Nurs 2003; 21: 92-95.
- 25. Winterborn RJ, Campbell WB, Heather BP, Earnshaw JJ. The management of short saphenous varicose veins: a survey of the members of the vascular surgical society of Great Britain and Ireland. Eur J Vasc Endovasc Surg 2004; 28: 400-403.
- 26. Myers KA, Zeng GH, Ziegenbein RW, Matthews PG. Duplex ultrasound scanning for chronic venous disease: recurrent varicose veins in the thigh after surgery to the long saphenous vein. Phlebology 1996; 11: 125-131.
- 27. El Wajeh Y, Giannoukas AD, Gulliford CJ, et al. Saphenofemoral venous channels associated with recurrent varicose veins are not neovascular. Eur J Vasc Endovasc Surg 2004; 28: 590-594.
- 28. Fassiadis N, Kianifard B, Holdstock JM, Whiteley MS. Ultrasound changes at the saphenofemoral junction and in the long saphenous vein during the first year after VNUS closure. Int Angiol 2002; 21: 272-274.





Abstract
Objective: To assess the efficacy of endovenous laser therapy (EVLT) for treating varicose veins with saphenous reflux.
Design: A trial of treatment, with results assessed by ultrasound surveillance.
Setting: Outpatient clinics with sonographer and nursing support.
Main outcome measures: Control of reflux; occlusion or obliteration of the saphenous veins assessed by ultrasound.
Results: EVLT was used to treat 404 veins in 308 patients. Univariate life table analysis showed primary success in 80% (95% CI, 69%–87%) and secondary success after further treatment of recurrent saphenous vein reflux by ultrasound-guided sclerotherapy in 88% (95% CI, 78%–95%) at 3 years. On multivariate Cox regression analysis, none of the covariates studied were associated with ultrasound failure.
Conclusions: Early results indicate that EVLT effectively controlled saphenous reflux. Its advantages are that it is performed as an outpatient procedure under local anaesthesia with immediate mobilisation, causes minimal disruption of activities, and avoids surgical trauma.