The high prevalence of vitamin D insufficiency among aged-care residents is well documented.1-3 Contributing factors include lack of exposure to sunlight,3 poor conversion in aged skin of pre-vitamin D3 to vitamin D3,4 and a paucity of vitamin D-rich dietary items. The undesirable effects of vitamin D deficiency include reduced bone mineral density,5 high bone turnover,6,7 and an increased risk of falls and fracture.3,8,9 Supplementation with vitamin D, its derivatives, or a combination of vitamin D and calcium increases bone mineral density,10,11 reduces the risk of hip and other fragility fractures,12-15 improves muscle function, and reduces falls among aged-care residents.3,16,17
Our study was designed to determine the feasibility of a program of administering an inexpensive preparation of high-dose vitamin D3 quarterly within a residential care setting. We used a similar regimen to that of Trivedi and coworkers14 who found that 4-monthly oral supplementation with 100 000 IU vitamin D3 given over 5 years reduced fractures in community-dwelling men and women aged over 65 years.
A total of 137 residents (107 treated, 30 control) from 11 facilities participated in the study (three facilities from Organisation A, and four each from B and C). Participants’ demographic characteristics are summarised in Box 1. At 6 months, 10 residents had died (seven treatment, three control), one subject from the treatment group was not in residence due to hospitalisation, and difficulties with venous access precluded blood testing in another.
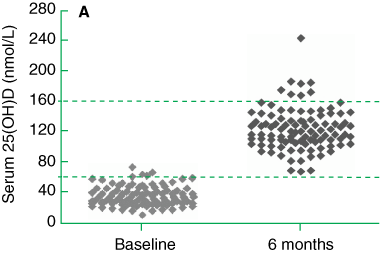
Baseline and 6-month serum 25(OH)D levels are shown in Box 1 and Box 2. The desirable range for serum 25(OH)D level, as defined by the IMVS laboratory, is 60–160 nmol/L. At baseline, the means of the control and treatment groups were similar. Combined, these groups yielded a baseline mean ± SD of 36.4 ± 12.7 nmol/L (range, 12–87 nmol/L; 95% CI of the mean, 34.2–38.5 nmol/L, with 130/137 (95%) residents being below the desirable range (< 60 nmol/L). According to Vieth’s classification,18 vitamin D deficiency was mild (25–49 nmol/L) in 98 (72%) subjects, moderate (12.5–24 nmol/L) in 17 (12%), and severe (< 12.5 nmol/L) in one subject (0.7%).
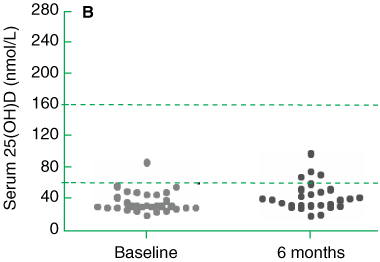
At 6 months, after the third dose of vitamin D3, mean serum 25(OH)D level for the treatment group had risen significantly (P < 0.0001; paired t test) with a change of mean from baseline of 87.6 nmol/L (95% CI, 81.5–92.1 nmol/L). At the same time, there was a small but statistically significant rise in serum 25(OH)D level in the control group (P < 0.02, paired t test), with a change of mean from baseline of 6.4 nmol/L (95% CI, 1.23–10.6 nmol/L). The difference between the means of the treatment and control groups at 6 months (81.2 nmol/L; 95% CI, 69.7–92.0 nmol/L) was highly significant (P < 0.001, unpaired t test). In the treatment group, the number of residents with serum 25(OH)D levels within the desirable range increased from 6 (6%) to 98 (100%). No resident was classified as deficient, and none had any adverse effects associated with dosing. The serum 25(OH)D level for one resident was 244 nmol/L — higher than the upper boundary of the desirable range, but well below toxic levels (> 690 nmol/L).19 In the control group, the number of residents with serum 25(OH)D levels within the desired range increased from one (3%) to four (15%). According to Vieth’s criteria,18 mild vitamin D deficiency persisted in 18 (67%) and moderate deficiency in two (7%) of these untreated residents.
The results of the substudies are summarised in Box 3, Box 4, and Box 5.
Substudy 1: All of the 31 residents who had 3-month trough levels of 25(OH)D analysed had values in the desired range (Box 3).
Substudy 2: Of 28 subjects treated at Organisation B, 25 survived to 6 months when vitamin D supplementation was discontinued. Of these, 18 had blood samples taken at 12 months (three had died, three refused further blood testing and another was too ill). Of the 44 subjects treated at Organisation C, 40 survived to 6 months when vitamin D supplementation was discontinued. Of these, 32 had blood samples taken at 12 months (three had died, three refused further testing and two were no longer in residence). In these 50 subjects, the mean serum 25(OH)D level at 12 months was below the desirable range (Box 4).
Substudy 3a and 3b: Of 35 subjects starting treatment at Organisation A (which continued the 3-monthly vitamin D supplemen-tation beyond 6 months), 33 survived to 6 months and 31 survived to 12 months. Of these, eight who had been in substudy 1 chose not to submit to further blood testing and another eight had ceased the regular supplement, leaving 15 available for testing at 12 months (Box 5). At 2 years, of these 15 subjects, two had died, one had been discharged and another was too agitated to provide a blood sample. This left 11 treated subjects. The mean serum 25(OH)D level was within the desired range, with no trend towards undesirable accumulation at both 12 and 24 months (Box 5).
Another potential criticism is the lack of co-supplementation with calcium. Based on the article by Chapuy et al12 and the interdependence of vitamin D and calcium metabolism, it can be argued that vitamin D3 should only be given with calcium, preferably in the same preparation. In support of combination formulations, it has been suggested that the serum 25(OH)D assay can be used as a surrogate for compliance with co-administered calcium. However, several factors need to be considered — the very different periodicity of dosing for vitamin D and calcium, differences in tolerance, and direct and indirect costs. Dosing frequency can be quarterly or less for vitamin D3, but calcium needs to be given daily, implying greater nursing demands.
We have shown that 3-monthly vitamin D3 is well tolerated. In contrast, inability to swallow large capsules and constipation are frequent, treatment-limiting effects of calcium supplementation. Indeed, lack of tolerance for the calcium component of combined vitamin D and calcium regimens has been invoked to explain the failure of recent community-based studies to show benefits from long-term supplementation with vitamin D with calcium.20 These studies contrast with the positive effect on fracture risk in the long-term community-based study of Trivedi et al,14 in which vitamin D3 was given without calcium. Our study shows that this inexpensive approach is also appropriate in residential aged-care.
1 Characteristics of the aged-care residents in the primary study, and serum 25-hydroxyvitamin D [25(OH)D] levels at baseline and at 6 months
2 Serum 25-hydroxyvitamin D [25(OH)D] level at baseline and at 6 months — A: treatment group; B: control group
4 Substudy 2: 25-hydroxyvitamin D [25(OH)D] level — supplementation for 6 months, no supplementation for 6 months (n = 50)
Received 11 January 2006, accepted 28 June 2006
- Alison E R Wigg1
- Caroline Prest1
- Peter Slobodian1
- Allan G Need2,3
- Leslie G Cleland1,3
- 1 Royal Adelaide Hospital, Adelaide, SA.
- 2 Institute of Medical and Veterinary Science, Adelaide, SA.
- 3 Department of Medicine, University of Adelaide, Adelaide, SA.
The study was supported by funding from the Australian Government Department of Health and Ageing through a National Arthritis and Musculoskeletal Conditions Improvements Grant. We would also like to acknowledge the generous support of staff and residents of Helping Hand Aged Care, Resthaven Incorporated and the Aged Care and Housing Group in South Australia.
None identified.
- 1. Morris HA, Morrison GW, Burr M, et al. Vitamin D and femoral neck fractures in elderly South Australian women. Med J Aust 1984; 140: 519-521.
- 2. Sambrook PN, Cameron ID, Cumming RG, et al. Vitamin D deficiency is common in frail institutionalised older people in northern Sydney [letter]. Med J Aust 2002; 176: 560. <MJA full text>
- 3. Flicker L, Mead K, MacInnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc 2003; 51: 1533-1538.
- 4. MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest 1985; 76: 1536-1538.
- 5. Outila TA, Karkkainen MU, Lamberg-Allardt CJ. Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: associations with forearm bone mineral density. Am J Clin Nutr 2001; 74: 206-210.
- 6. Kipen E, Helme RD, Wark JD, et al. Bone density, vitamin D nutrition, and parathyroid hormone levels in women with dementia. J Am Geriatr Soc 1995; 43: 1088-1091.
- 7. Jesudason D, Need AG, Horowitz M, Flicker L. Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone 2002; 31: 626-630.
- 8. Diamond T, Smerdely P, Kormas N, et al. Hip fracture in elderly men: the importance of subclinical vitamin D deficiency and hypogonadism. Med J Aust 1998; 169: 138-141.
- 9. Weatherall M. A meta-analysis of 25 hydroxyvitamin D in older people with fracture of the proximal femur. N Z Med J 2000; 113: 137-140.
- 10. Parfitt A. Osteomalacia and related disorders. In: Avioli LV, Krane SM, editors. Metabolic bone disease and clinically related disorders. Philadelphia: WB Saunders Company, 1990: 329-396.
- 11. Mithal A, Sen I, Naik V, et al. Spectacular recovery of bone mineral density following treatment of osteomalacia [abstract]. J Bone Miner Res 2000; 15: S577.
- 12. Chapuy M, Arlot M, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992; 327: 1637-1642.
- 13. Heikinheimo RJ, Inkovaara JA, Harju EJ, et al. Annual injection of vitamin D and fractures of aged bones. Calcif Tissue Int 1992; 51: 105-110.
- 14. Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003; 326: 469-472.
- 15. Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 2005; 293: 2257-2264.
- 16. Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res 2003; 18: 343-351.
- 17. Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA 2004; 291: 1999-2006.
- 18. Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999; 69: 842-856.
- 19. Mason RS, Posen S. The relevance of 25-hydroxycalciferol measurements in the treatment of hypoparathyroidism. Clin Endocrinol (Oxf) 1979; 10: 265-269.
- 20. Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium OR vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 2005; 365: 1621-1628.





Abstract
Objective: To assess the feasibility of administering an inexpensive preparation of vitamin D3 100 000 IU orally 3 monthly to aged-care residents.
Design: Prospective, controlled open-label implementation trial.
Setting: Residential aged care, November 2003 to May 2004 (primary study).
Participants: 137 ambulant residents: 107 treated (mean age, 85 years; 79 were women), 30 untreated controls (mean age, 87 years; 22 were women).
Interventions: Lactose microencapsulated vitamin D3 100 000 IU orally at baseline, then 3 monthly (three or more doses); untreated subjects were observed contemporaneously.
Main outcome measures: Serum levels of 25-hydroxyvitamin D [25(OH)D] at 6 months compared with baseline; acceptability of the program to residents and staff.
Results: At baseline, 95% of residents assessed (n = 137) had serum 25(OH)D levels below the desirable range of 60–160 nmol/L. At 6 months, all treated residents (n = 98) achieved desired levels, with the mean (± SD) 25(OH)D level increasing from 36.4 ± 12.6 nmol/L (range, 12–75 nmol/L) at baseline to 124.0 ± 27.9 nmol/L (range, 68–244 nmol/L). In no resident did 25(OH)D approach toxic levels. The mean serum 25(OH)D level remained low in the control group (n = 27): 42.8 ± 18.3 nmol/L (range, 18–98 nmol/L). The difference between the mean 25(OH)D levels of treatment and control groups at 6 months was 81.2 nmol/L (95% CI, 69.7–92.0 nmol/L). The cost of the supplement was $4 per resident per annum. Substudies showed mean trough serum 25(OH)D levels in the desired range at 3 months (n = 31), but below the desired range at 6 months (n = 50). Subjects given 3-monthly doses for up to 2 years maintained serum 25(OH)D levels within the desired range, with no trend toward undesirable accumulation (n = 11).
Conclusions: Vitamin D3 100 000 IU given orally 3 monthly is a practical, safe, effective and inexpensive way to meet the vitamin D3 requirements of aged-care residents.