For decades, adjuvant therapy with the partial oestrogen agonist, tamoxifen, has been known to reduce the incidence of contralateral breast cancer in women with early breast cancer. This knowledge provided the rationale for its evaluation in primary prevention in women at high risk. Four trials examining the use of tamoxifen in this setting have reported their initial results and, together, have convincingly shown that it has a role in such prevention.1 An overview of these trials found that about half of oestrogen-receptor-positive tumours were prevented with tamoxifen, but there was little effect on receptor-negative tumours (where a non-significant increase was shown).1 Venous thromboembolic events (VTEs) were increased two-fold, and endometrial cancer by more than two-fold in women using tamoxifen. The benefits were similar in women under and over the age of 50, as was the relative risk for VTEs. However, the absolute increase in VTEs was greater in older women, and almost all endometrial cancers occurred in post-menopausal women, suggesting that tamoxifen for preventing breast cancer has the highest benefit-to-risk ratio in women at high risk of breast cancer who are aged 45–50 years. The absolute risks and benefits for 1000 50-year-old women followed up for 5 years are shown in Box 1. An overall assessment of the role of tamoxifen in prevention will depend strongly on the next 5 years of follow-up; the first of the two IBIS (International Breast Cancer Intervention Study) trials, in which Australia has collaborated, is continuing to follow up all women in a blinded fashion.
A recent study comparing blinded treatment using raloxifene (the other well known selective oestrogen-receptor modulator) or placebo, with breast cancer incidence as the primary endpoint, reported a 59% reduction in invasive cancer2 and confirmed earlier data that raloxifene is likely to be at least as effective as tamoxifen in preventing new cancers.3 In addition, no excess of gynaecological events was seen with raloxifene, but the incidence of VTEs was similar to that seen with tamoxifen. A large American study of 19 000 women at high risk of breast cancer, which directly compared raloxifene with tamoxifen, is due to report in late 2006, and should provide definitive information on this promising compound (see <http://www.breastcancerprevention.org>).
Substantial data are now showing greater efficacy than tamoxifen for each of the three third-generation aromatase inhibitors (anastrozole, letrozole, exemestane) in the adjuvant setting for post-menopausal women with hormone-receptor-positive tumours.4-7 Aromatase inhibitor therapy results in even greater reductions in the incidence of new contralateral tumours than tamoxifen,3 and side effects are generally fewer than those experienced with tamoxifen, except that the aromatases reduce bone density and increase fracture rates.4 This suggests a strong potential for aromatase inhibitors in preventing breast cancer, and has led to the development of the second IBIS prevention trial comparing anastrozole with placebo in 6000 post-menopausal women at high risk of breast cancer (see <http://www.ibis-trials.org>). Another trial evaluating exemestane in breast cancer prevention is also under way.
Consistent reports of a beneficial effect of aspirin8 and, to a lesser extent, cyclo-oxygenase-2 (COX-2) inhibitors generated interest in using a COX-2 inhibitor for prevention, especially for premenopausal women, where there were no current trials. Further work in this area awaits a decision on the long-term safety of COX-2 inhibitors.
Technological advances have expanded the clinical application of ultrasound beyond its traditional role as an adjunct to mammo-graphy (Box 2). High-resolution ultrasound detects very small lesions, and more reliably differentiates malignant from benign solid masses.13,14 However, it also identifies many benign lesions that usually necessitate further investigation. This has been the major limitation of ultrasound in studies that have evaluated its accuracy in screening,15 the incremental false positive rate being around 5%–7% if ultrasound is used as an adjunct to screening mammography.15 Ultrasound remains unproven as a screening tool, and its potential role in screening is likely to be limited to women with dense breasts on mammography where it can detect cancers not visible on mammography.15
Percutaneous core needle biopsy (CNB) methods are used to evaluate screen-detected or symptomatic lesions, allowing a definitive histological diagnosis to be established (Box 3). Internationally, CNB has widely replaced cytology obtained by fine needle aspiration biopsy (FNAB), and is the established method of sampling image-detected lesions. The choice of whether to use CNB or FNAB is generally guided by the type of lesion being sampled and local expertise. CNB provides more information than FNAB16 and assists in planning therapy before surgery in women with cancer, as it reliably distinguishes between invasive and in-situ disease, and allows determination of hormone and growth factor receptors. Ultrasound-guided CNB, in particular, has been shown to have high accuracy,17 and is superior to freehand biopsy and cost-effective in establishing a histological diagnosis.18 CNB diagnosis also allows women with benign conditions to obtain a specific diagnosis and the choice of avoiding surgery. FNAB has the advantage that it can be reported immediately and may increase overall sensitivity when added to CNB.
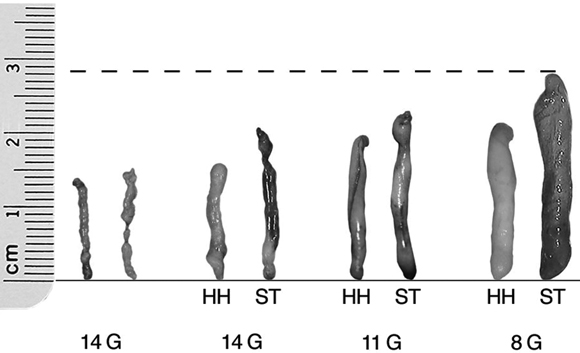
Directional vacuum-assisted large-gauge CNB has been introduced into practice in recent years because it acquires larger samples of tissue (relative to automated core biopsy) and potentially avoids under-sampling a lesion (it reduces false negative results).11,19 This is particularly important where there may be a spectrum of change ranging from atypia to in-situ cancer (eg, sampling of screen-detected calcification) and when sampling masses smaller than 1.5 cm.11 Vacuum-assisted large-gauge core biopsy devices can also be used to remove selected benign (or low risk) lesions,20 although this is not done in Australian practice outside of clinical research because its long-term efficacy has not been established.
Digital mammography (DM) has replaced conventional (film-screen) mammography in some breast screening services, predominantly in the United States and Europe. While it may not have major clinical advantages over conventional mammography, DM detects additional cancers,15 although this may be counterbalanced by a higher recall rate (more false-positive results).21 Recent evidence indicates that DM may be significantly more sensitive than film mammography in screening women who have dense breasts and women who are younger than 50 years or are premenopausal or perimenopausal.22 Potential advantages of DM include the use of computer-aided detection (algorithm-based computer programs that alert the radiologist to possible abnormalities on the mammogram), and allowing centralised film reading. Both these aspects of DM provide potential workforce and service-provision solutions for breast screening that are particularly relevant to Australia, and BreastScreen Victoria is currently exploring the use of DM in screening.
evaluation of the integrity of breast prostheses;
determining the presence of any breast disease in women with proven malignant axillary nodes and normal mammography and ultrasound; and
evaluating extent of disease (multifocality) where this cannot be reliably excluded on conventional imaging.12,23
Ongoing studies should identify those women with most to gain from this investigation.
Interest in breast MRI in recent years has largely focused on its role in screening women with inherited mutations of the breast cancer genes (BRCA1 or BRCA2). Although these women make up a small proportion (about 5%–10%) of all women with breast cancer, they have the highest lifetime risk of developing the disease. Mammography has very poor sensitivity for cancer detection in women with gene mutations, detecting fewer than half of the breast cancers that develop in this group (with about half presenting as clinical “interval” cancers). Data from non-randomised studies show that MRI detects cancers not identified by mammography in these women,15,24,25 with the most recent data from a United Kingdom trial showing MRI to have a much higher sensitivity than mammography (77% v 40%; P = 0.01), but with significantly lower specificity.24 It is unknown whether this improved sensitivity reported in non-randomised (comparative) studies will translate into a reduction in breast cancer mortality — the important outcome measure in cancer-screening trials. MRI breast screening is likely to be restricted for some time to women with known or suspected gene mutations (or those at substantially elevated risk of developing breast cancer), but its true efficacy as a screening test remains unclear.
Axillary lymph node status is the most significant individual prognostic factor in patients with breast cancer.26 Knowledge of nodal status is important in deciding on both adjuvant systemic and locoregional treatments. Standard management of the axilla has, until recently, been axillary node dissection, but this procedure is associated with significant morbidity and provides no benefit for most women with breast cancer who do not have axillary lymph node involvement. Pathological assessment of axillary lymph nodes remains the only reliable means of assessing nodal involvement.26 Ultrasound of the axilla can identify enlarged or abnormal nodes in up to 50% of patients with breast cancer,27 and fine needle aspiration cytology or core biopsy of these nodes identifies up to 40% of patients with involved axillary nodes.27
Sentinel lymph node (SLN) biopsy has emerged as an accurate, minimally invasive procedure to assess axillary lymph node status in patients without evidence of nodal disease on clinical or ultrasound assessment.28 An SLN is any node or nodes receiving lymphatic drainage from a primary tumour site. Localisation of SLNs in the breast is performed by injecting blue dye and radioactive colloid tracer into the breast.29 These substances pass through regional lymphatic vessels to the SLNs which are detectable because they are visibly blue, or because radioactivity is detected in the nodes by a handheld probe.28 The combination of blue dye and radioisotope gives better results than either agent alone. Localisation rates of over 95% are possible, with the highest rate of node detection (> 99%) coming from injection in the subareolar region.28,30 Rates of false negative results are now less than 5%. SLN biopsy causes significantly less morbidity than axillary dissection, and recovery is quicker. Demand on resources is less, and there are reductions in operation time, drain use, and hospital stay.31 Randomised studies have shown that SLN biopsy is a safe and effective alternative to axillary node dissection for nodal staging in patients with clinically node-negative early breast cancer,31,32 and is associated with reduced arm morbidity and better quality of life than axillary dissection.32
Breast reconstruction, using prostheses or autologous tissue, is safe and has no impact on detection of recurrence or overall survival.33 Immediate reconstruction has economic benefits, produces better results than delayed reconstruction and reduces the psychological morbidity associated with mastectomy.34 Recent advances include the increasing use of skin-sparing mastectomy (which removes the breast tissue but preserves most of the breast skin) combined with immediate breast reconstruction using a latissimus dorsi (LD) flap or a transverse rectus abdominus myocutaneous (TRAM) flap. Other improvements include extending the dissection to include fat over the LD muscle (the so-called extended LD flap; Box 4) so that an implant is not required, and raising the lower abdominal fat and skin on the deep inferior epigastric vessels (the so-called diep flap) alone, thereby sparing the rectus muscle.
Most patients with breast cancer receive systemic therapy. This is usually given after surgery (adjuvant therapy), but women with large operable or locally advanced tumours may be offered systemic therapy as an initial treatment before surgery (neoadjuvant therapy). Neoadjuvant systemic therapy does not confer any additional survival benefit over adjuvant systemic therapy and is primarily used to reduce tumour bulk. Neoadjuvant chemotherapy has been widely used in patients with large operable breast cancers, but there have been relatively few studies of neoadjuvant endocrine therapy. Initial results with neoadjuvant hormone therapy have been impressive, with a median 80% reduction in tumour volume achieved with 3 months of therapy with the aromatase inhibitor, letrozole.35 In one small study, 117 postmenopausal women with cancers which were oestrogen-receptor-positive and/or progesterone-receptor-positive were randomly assigned to either neoadjuvant chemotherapy or hormone therapy.36 Clinical response rates, and rates of response observable by mammo-graphy and pathological testing were identical for both chemotherapy and hormone therapy with either anastrozole or exemestane. Neutropenia, neuropathy and alopecia were common in women treated with chemotherapy, while those receiving endocrine therapy reported hot flushes, fatigue and arthralgia. The rate of breast-conserving surgery was higher after neoadjuvant endocrine therapy, although this difference did not reach statistical significance (P = 0.054).
The new aromatase inhibitors have significant advantages over tamoxifen in the neoadjuvant setting. In one randomised study letrozole produced a higher clinical response rate than tamoxifen (55% v 36%; P < 0.001),37 and higher breast conservation rates (45% v 35%; P = 0.022). A higher rate of conversion from mastectomy to breast conservation surgery with aromatase inhibitors compared with tamoxifen has also been shown in trials of anastrozole and exemestane. Patients whose tumours expressed only low levels of oestrogen receptor and those that overexpressed the growth factor receptors HER1 and/or HER2, which are usually associated with a worse prognosis, had a significantly greater chance of responding to letrozole than to tamoxifen.37
Neoadjuvant endocrine therapy can be used in many postmenopausal women with larger tumours to avoid mastectomy, and to render operable those tumours that are locally advanced at diagnosis and not suitable for surgery. The optimal duration of treatment remains unclear, but current evidence suggests that tumour shrinkage with agents such as letrozole continues beyond 3–4 months, and treatment can be continued until the cancer has shrunk sufficiently either to become operable or to allow breast-conserving surgery (Box 5).
Two studies of women with HER2-positive early breast cancer, treated by combination chemotherapy and randomly assigned to receive weekly trastuzumab for 1 year or no further treatment, reported that disease-free survival (DFS) was significantly improved by trastuzumab (85.3% v 67.1% at 4 years; hazard ratio [HR], 0.48; P = 3 × 10-12), as was overall survival (HR, 0.67; P = 0.015).38 The clinical benefits of trastuzumab were further confirmed in a large European trial that randomly assigned women who had completed chemotherapy to receive trastuzumab for 1 or 2 years, or to receive no further treatment.39 Women who had received trastuzumab had a significant improvement in DFS (DFS at 2 years, 85.8% v 77.4%; HR, 0.54; P < 0.0001), but experienced a small and significant deterioration in left ventricular function (7.08% v 2.21%; P < 0.001).39 These recent data indicate that trastuzumab should be considered for adjuvant therapy in women whose tumours overexpress HER2. Further long-term safety data are required before its use becomes routine in all but women with breast cancer associated with poor prognostic features.
1 Primary prevention of breast cancer with tamoxifen: outcomes in 1000 women at high risk of breast cancer followed for 5 years
2 Breast ultrasound in diagnosis
In the symptomatic or clinical setting, breast ultrasound has emerged as a key diagnostic test in:
Evaluating breast symptoms in young women9 and in women who are pregnant or lactating, where it has better accuracy than mammography.
Adjunct imaging of women with palpable lumps (irrespective of age), particularly where the breasts are relatively dense (contain a large amount of glandular tissue) on mammography — combining mammography and ultrasound in women with clinical findings improves diagnostic accuracy (2%–4% of symptomatic breast cancers are missed when both imaging tests are negative,10 whereas about 10%–20% of breast cancers are not correctly diagnosed if mammography alone is used).
Image-guided intervention11 — ultrasound is used in preference to mammography (for both palpable and image-detected lesions) because it is a more efficient guidance method, avoids radiation, is generally more acceptable to the patient, and is more accessible to clinician users.
Women with breast cancer — ultrasound is routinely performed as part of preoperative assessment (unless the breast tissue is fat-replaced on mammography), where it contributes information on the extent of disease within the affected breast (it may identify additional foci of cancer) and the axilla, and may also detect mammographically-occult cancer in the contralateral breast.12
3 Core needle biopsy samples from conventional (automated) biopsy and vacuum-assisted biopsy methods and increasing core needle gauge
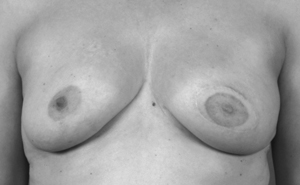
5 Use of neoadjuvant endocrine therapy in tumour shrinkage and breast conservation
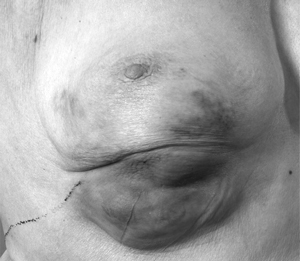 Locally advanced right breast cancer. |
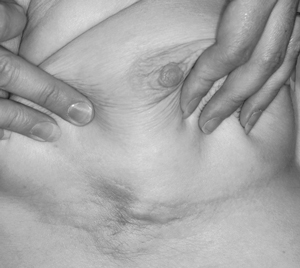 After 9 months neoadjuvant endocrine therapy with letrozole. |
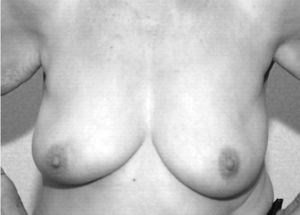 After conservative surgery to the right breast. |
Photos provided by J M Dixon.
- Nehmat Houssami1
- Jack Cuzick2
- J Michael Dixon3
- 1 Screening and Test Evaluation Program, School of Public Health, University of Sydney, Sydney, NSW.
- 2 Cancer Research UK, Centre for Epidemiology, Mathematics and Statistics, Wolfson Institute of Preventive Medicine, London, UK.
- 3 Edinburgh Breast Unit, Western General Hospital, Edinburgh, Scotland.
Jack Cuzick is a consultant for AstraZeneca and is on the advisory boards of Eli Lilly and Pfizer; Michael Dixon has received honoraria for lectures and educational grants for research from AstraZeneca, Novartis and Pfizer.
- 1. Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast cancer prevention trials. Lancet 2003; 361: 296-300.
- 2. Martino S, Cauley JA, Barrett-Connor E, et al. CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 2004; 96: 1751-1761.
- 3. Cuzick J. Aromatase inhibitors for breast cancer prevention. J Clin Oncol 2005; 23: 1636-1643.
- 4. Howell A, Cuzick J, Baum M, et al. ATAC Trialists’ Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005; 365: 60-62.
- 5. Coombes RC, Hall E, Gibson LJ, et al. Intergroup Exemestane Study: a randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 2004; 350: 1081-1092.
- 6. Jakesz R, Kaufmann M, Gnant M, et al. Benefits of switching postmenopausal women with hormone-sensitive early breast cancer to anastrozole after 2 years adjuvant tamoxifen: combined results from 3123 women enrolled in the ABCSG Trial 8 and the ARNO 95 Trial. Breast Cancer Res Treat 2004; 88 Suppl 1: S7.
- 7. Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003; 349: 1793-1802.
- 8. Garcia-Rodriguez LA, Gonzalez-Perez A. Risk of breast cancer among users of aspirin and other anti-inflammatory drugs. Br J Cancer 2004; 91: 525-529.
- 9. Houssami N, Irwig L, Simpson JM, et al. Sydney Breast Imaging Accuracy Study: comparative sensitivity and specificity of mammography and sonography in young women with symptoms. AJR Am J Roentgenol 2003; 180: 935-940.
- 10. Houssami N, Irwig L, Loy C. The accuracy of combined breast imaging in young women. Breast 2002; 11: 36-40.
- 11. Parker SH. Ultrasound-guided needle procedures in the breast. In: Stavros AT. Breast ultrasound. Philadelphia: Lippincott Williams & Wilkins, 2004: 742-777.
- 12. Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US and MR imaging in pre-operative assessment of breast cancer. Radiology 2004; 233: 830-849.
- 13. Stavros AT, Thickman D, Rapp CL, et al. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology 1995; 196: 123-134.
- 14. Rahbar G, Sie AC, Hansen GC, et al. Benign versus malignant solid breast masses: US differentiation. Radiology 1999; 213: 889-894.
- 15. Irwig L, Houssami N, van Vliet C. New technologies in screening for breast cancer: a systematic review of their accuracy. Br J Cancer 2004; 90: 2118-2122.
- 16. Britton PD. Fine needle aspiration or core biopsy. Breast 1999; 8: 1-4.
- 17. Parker SH, Jobe WE, Dennis MA, et al. US-guided automated large-core breast biopsy. Radiology 1993; 187: 507-511.
- 18. Liberman L, Feng TL, Dershaw DD, et al. Ultrasound-guided core breast biopsy: utility and cost-effectiveness. Radiology 1998; 208: 717-723.
- 19. Liberman L. Clinical management issues in percutaneous core biopsy. Radiol Clin North Am 2000; 38: 791-807.
- 20. Fine RE, Whitworth PW, Kim JA, et al. Low-risk palpable breast masses removed using a vacuum-assisted hand-held device. Am J Surg 2003; 186: 362-367.
- 21. Skaane P, Skjennald A. Screen-film mammography versus full-field digital mammography with soft-copy reading: randomised trial in a population-based screening program — the Oslo II study. Radiology 2004; 232: 197-204.
- 22. Pisano ED, Gatsonis C, Hendrick E, et al, for the Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group. Diagnostic performance of digital versus film mammography for breast cancer screening. N Engl J Med 2005; 353: 1773-1783.
- 23. Liberman L, Morris EA, Dershaw DD, et al. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. AJR Am J Roentgenol 2003; 180: 901-910.
- 24. MARIBS study group. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 2005; 365: 1769-1778.
- 25. Liberman L. Breast cancer screening with MRI — what are the data for patients at high risk? N Engl J Med 2004; 351: 497-500.
- 26. Veronesi U, Galiberti V, Zurrida S, et al. Prognostic significance of number and level of axillary node metastases in breast cancer. Breast 1993; 2: 224-228.
- 27. Kuenen-Boumeester V, Menke-Pluymers M, de Kanter AY, et al. Ultrasound-guided fine needle aspiration cytology of axillary lymph nodes in breast cancer patients: a preoperative staging procedure. Eur J Cancer 2003; 39: 170-174.
- 28. Allweis TM, Badriyyah M, Bar Ad V, et al. Current controversies in sentinel lymph node biopsy for breast cancer. Breast 2003; 12: 163-171.
- 29. Derossis AM, Fey J, Yeung H, et al. A trend analysis of the relative value of blue dye and isotope localization in 2000 consecutive cases of sentinel lymph node biopsy for breast cancer. J Am Coll Surg 2001; 193: 473-478.
- 30. Chagpar A, Martin RC 3rd, Chao C, et al. Validation of subareolar and periareolar injection techniques for breast sentinel lymph node biopsy. Arch Surg 2004; 139: 614-618.
- 31. Veronesi U, Paganelli G, Viale G, et al. A randomised comparison of sentinel-node biopsy with routine dissection in breast cancer. N Engl J Med 2003; 349: 546-553.
- 32. Fallowfield LJ, Fleissig A, Langridge CL, et al, on behalf of the ALMANAC Trialists. Age related quality of life (QoL) benefits: results from the Almanac Trial with 18 months follow-up. Eur J Cancer Suppl 2005; 3: 39. (Abstracts from the 9th Nottingham International Breast Cancer Conference.)
- 33. Fine NA , Mustoe TA. Breast reconstruction. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the breast. Philadelphia: Lippincott Williams & Wilkins, 2005: 801-818.
- 34. Khoo A, Kroll SS, Reece GP, et al. A comparison of resource costs of immediate and delayed breast reconstruction. Plast Reconstr Surg 1998; 101: 964-968.
- 35. Dixon JM, Anderson TJ, Miller WR. Neoadjuvant endocrine therapy of breast cancer: a surgical perspective. Eur J Cancer 2002; 38: 2214-2221.
- 36. Semiglazov VF, Semiglazov V, Ivanov V, et al. Preoperative hormonal therapy vs chemotherapy in postmenopausal ER-positive breast cancer patients. Eur J of Cancer Suppl 2004; 2: 71.
- 37. Eierman W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of post-menopausal breast cancer patients with letrozole: a randomised double blind multicentre study. Ann Oncol 2001; 12: 1527-1532.
- 38. Romond EH, Perez E, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353: 1673-1684.
- 39. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al, for the Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353: 1659-1672.





Abstract
The reduction in the incidence of contralateral breast cancer in women treated with adjuvant tamoxifen provided a model for prevention using endocrine agents. Oestrogen-receptor-positive cancer can be prevented with tamoxifen, but side effects limit its clinical utility, and the risk–benefit ratio is not sufficiently high to routinely recommend tamoxifen as a preventive agent.
Agents being evaluated in prevention trials include raloxifene and the aromatase inhibitors; these are expected to be at least as effective as tamoxifen and to have fewer side effects.
Core needle biopsy (providing histological information) and high-resolution breast ultrasound enhance preoperative assessment of breast cancer.
Mammography remains the only screening test shown to reduce breast cancer deaths in randomised trials. Magnetic resonance imaging may have a role in screening women with inherited mutations of the breast cancer genes.
Sentinel lymph node biopsy accurately assesses lymph node status and is associated with less morbidity than axillary dissection. Where the biopsy is negative (no histologic evidence of metastases), no further axillary treatment is necessary.
Breast reconstruction after mastectomy can produce good cosmetic results, especially where autologous tissue is used. Myocutaneous flaps using latissimus dorsi or transverse rectus abdominus muscles are increasingly popular.
Adjuvant trastuzumab therapy in patients whose tumours overexpress HER2 (growth factor receptor) can reduce recurrence rates and improve survival.
Neoadjuvant endocrine therapy (as an initial treatment before surgery) is an underutilised treatment in postmenopausal women with oestrogen-receptor-positive large operable or locally advanced cancers. It makes more patients suitable for surgery and offers others the choice of breast conservation.