Australian Aboriginals and Torres Strait Islanders are burdened with higher rates of many chronic illnesses, most notably diabetes, vascular disease and renal failure.1 However, there is limited information regarding epilepsy in these populations.
Population-based studies have shown higher incidences of epilepsy in developing countries, with the age-adjusted incidence ranging from 64 to 190 per 100 000 person-years,2-6 while that in developed countries is lower, at 24 to 53 per 100 000 person-years.7-10 However, no direct comparative studies have been performed. The most commonly cited hypothesis to explain this incidence difference is a higher rate of infectious diseases in developing countries (eg, those causing cerebral tuberculosis or neurocysticercosis).11,12
The health status of Australia’s Indigenous population is in many respects more typical of that in developing countries.13 However, in contrast to the situation in many developing nations, Indigenous Australians have a full range of health care services available to them, including a comprehensive range of neurological investigations — neurological assessment, electroencephalography (EEG), and cerebral imaging, including computed tomography (CT) and magnetic resonance imaging (MRI). However, geographic isolation and cultural issues lead to persisting inequalities in health care utilisation. Government strategies attempt to overcome this through subsidised transport from remote regions to major centres, and “outreach” medical clinics, where doctors visit remote communities by plane.
Cairns Base Hospital (CBH) in Far North Queensland serves a vast and mostly sparsely populated area about the same size as Victoria or the United Kingdom. Of the population of 230 000, of whom 135 000 live in Cairns and its immediate surrounds, 13% are Indigenous.14 The hospital’s catchment area extends to the Torres Strait, 1000 km away. The only specialist neurological services for the region are at CBH, which also provides the only EEG, CT and MRI services. These are available free of charge to all patients.
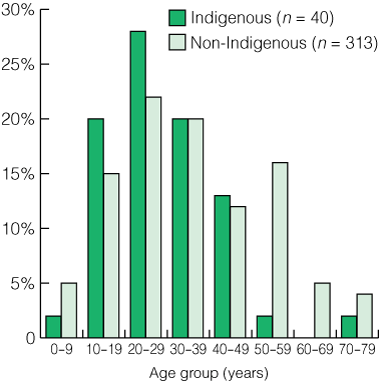
From 1 September 2002 to 31 March 2005, 359 patients were seen in the epilepsy clinic, of whom 11% (n = 40) were Indigenous, 87% (n = 313) non-Indigenous, and 2% (n = 6) of unknown status. Over a similar period, 13% (118/918) of the EEGs performed were on Indigenous patients. These figures compare favourably with the expected proportion of Indigenous people in the catchment population. Indigenous patients attending the clinic were significantly younger (P = 0.025, unpaired t test), with a pronounced peak between the ages of 10 and 30 years. Non-Indigenous patients attending the clinic were spread more evenly across the age spectrum (Box 1); 53% of both Indigenous and non-Indigenous patients attending the clinic were female.
Non-Indigenous patients (compared with Indigenous patients) presenting to the epilepsy clinic were more likely to receive a diagnosis of a non-epileptic disorder (31% v 18%), although this difference was not significant. Non-epileptic attacks included syncope, panic attacks and pseudoseizures. However, converging results come from the finding that non-Indigenous patients were more likely to have normal EEG results (58% v 47%; P = 0.025; Fisher’s exact χ2 test) (Box 2).
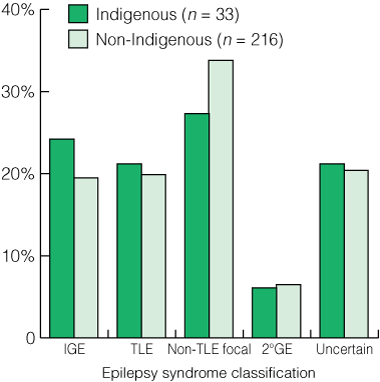
For patients attending the epilepsy clinic who were diagnosed with epilepsy, the proportions of Indigenous and non-Indigenous patients in the main syndrome groupings were remarkably similar. In a parallel finding, Indigenous and non-Indigenous patients with abnormal EEG findings showed similar proportions of the different abnormalities (Box 3). A crude estimation of seizure burden was calculated by adding annual seizure frequency for each seizure type for each patient. This can be quite inaccurate for some seizures, such as absences, which may not always be detected, and myoclonic seizures which may be erratic, frequent and occur in clusters. Within these limitations, no significant difference in seizure burden was noted between Indigenous and non-Indigenous patients.
Sixty per cent of Indigenous patients and 56% of non-Indigenous patients attending the clinic had cerebral imaging performed or already available. Reasons for not performing imaging included non-epileptic attacks, idiopathic generalised epilepsy, or patient non-compliance. Twenty per cent of Indigenous patients and 17% of non-Indigenous patients were found to have abnormalities on CT or MRI scans that could account for their epilepsy syndrome (Box 4). Epileptogenic abnormalities in both groups included ischaemic injury, tumour, dysplasia, hippocampal sclerosis and post-traumatic gliosis. That there was no significant difference in the rate of reported head injury (13% non-Indigenous patients v 18% Indigenous) collaborated these findings. Alcohol was consumed by 38% of non-Indigenous and 37% of Indigenous patients with epilepsy (Box 5). There was no significant difference in the amount of alcohol being consumed among those currently drinking, although subject numbers were small.
It is a commonly held belief among those working with Indigenous people that most cases of epilepsy are due to the effects of alcohol excess and head trauma. Our findings of similar rates of epileptogenic abnormalities seen on structural imaging provide no evidence to support this belief. The rate of structural abnormalities is slightly higher than that seen in series from first seizure clinics (about 13%),15 but this is probably accounted for by the mixture of patients in our study, which includes those with refractory epilepsy in whom brain lesions are more commonly observed. A very similar percentage of Indigenous and non-Indigenous patients presenting to the epilepsy clinic consumed alcohol. It is not possible to say that alcohol does not contribute to epilepsy in Indigenous patients, as our study is not primarily designed to answer this question, but our data suggest that alcohol is relevant in a minority of Indigenous patients with epilepsy.
Of the several possible reasons for the more severe epilepsy presentations in Indigenous patients, the most likely relate to inequalities in health care utilisation. Indigenous Australians, for a variety of reasons including cultural issues and geographic isolation, are less likely to consult a medical practitioner.16,17 Despite recent attempts to improve relationships between Indigenous Australians and government bodies, a history of mistrust leads to reluctance to seek help. Added to this are fewer doctors and often only a fly-in service in more remote regions. In some instances, there seems to be a “learned helplessness”, perhaps tied in with low socioeconomic status and lowered expectations from life. Some Indigenous Australians simply put up with medical conditions, such as intermittent seizures, that their non-Indigenous compatriots would seek help for. This may also be related to reduced medication compliance, but this is a complex issue. Reduced compliance can result from inadequate explanations from medical staff, and financial or geographical difficulty in obtaining medication.
Racial variation in epilepsy has been examined in the United States, which appears to show a modestly increased (1.7 times higher) incidence of epilepsy in African American children.18 The same study also examined the effect of socioeconomic class, showing that, after controlling for race, the incidence of epilepsy remained significantly higher in people of lower socioeconomic status.18 Similar socioeconomic effects have been reported in the UK, where a 2.3-fold increase in epilepsy incidence was seen in the lowest fifth compared with the highest fifth of the socioeconomic spectrum.19 It is unclear whether this phenomenon reflects cause, effect, or an epiphenomenon.
2 Classification of patients attending the electroencephalography (EEG) service (Sep 2002 – Mar 2005)
Indigenous patients were more likely to have an abnormal EEG (P = 0.025, Fisher’s exact χ2 test). |
|||||||||||||||
3 Classification of patients presenting to the epilepsy clinic (excluding non-epileptic presentations)
4 Proportion of patients treated in the epilepsy clinic in whom abnormalities* were detected on CT or MR imaging of the brain
Received 26 February 2006, accepted 13 April 2006
- John Archer1,2
- Ruth Bunby2
- 1 School of Medicine, James Cook University, Cairns, QLD.
- 2 Department of Neurology, Cairns Base Hospital, Cairns, QLD.
We thank Kate Heath (clinical benchmarking) and Desmond Letcher (data manager, Emergency Department), Cairns Base Hospital.
None identified.
- 1. Singh G, Hoy W. Kidney volume, blood pressure and albuminuria: findings in an Australian Aboriginal community. Am J Kidney Dis 2004; 43: 254-259.
- 2. Brewis M, Poskanzer D, Rolland C, Miller H. Neurologic disease in an English city. Acta Neurol Scand 1966; 42 Suppl 24: 1-89.
- 3. De Graff A. Epidemiologic aspects of epilepsy in Norway. Epilepsia 1974; 15: 291-299.
- 4. Granieri E, Rosati G, Tola R, et al. A descriptive study of epilepsy in the district of Copparo, Italy, 1964-1978. Epilepsia 1983; 24: 502-514.
- 5. Hauser W, Annegers J, Kurland L. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia 1993; 34: 453-468.
- 6. Lavados J, Germain L, Morales A, et al. A descriptive study of epilepsy in the district of El Salvador, Chile, 1984-1988. Acta Neurol Scand 1992; 85: 249-256.
- 7. Placencia M, Shorvon S, Paredes V, et al. Epileptic seizures in an Andean region of Ecuador: incidence and prevalence and regional variation. Brain 1992; 115(Pt 3): 771-782.
- 8. Placencia M, Sander J, Roman M, et al. The characteristics of epilepsy in a largely untreated population in rural Ecuador. J Neurol Neurosurg Psychiatry 1994; 57: 320-325.
- 9. Rwiza H, Kilonzo G, Haule J, et al. Prevalence and incidence of epilepsy in Ulanga, a rural Tanzanian district: a community-based study. Epilepsia 1992; 33: 1051-1056.
- 10. Tekle-Haimanot R, Forsgren L, Ekstedt J. Incidence of epilepsy in rural central Ethiopia. Epilepsia 1997; 38: 541-546.
- 11. Senanayake N, Roman GC. Epidemiology of epilepsy in developing countries. Bull World Health Organ 1993; 71: 247-258.
- 12. Pal D, Carpio A, Sander J. Neurocysticercosis and epilepsy in developing countries. J Neurol Neurosurg Psychiatry 2000; 68: 137-143.
- 13. Australian Medical Association. Public report card 2002. Aboriginal and Torres Strait Islander Health. No more excuses. Available at: http://www.ama.com.au/web.nsf/doc/WEEN-5GB4BW/$file/Indigenous%20Report.pdf(accessed May 2006).
- 14. MapInfo Australia, Australian Bureau of Statistics. CDATA 2001 (a database of the Australian 2001 census) — full GIS, release 2. Canberra: MapInfo/ABS, 2002.
- 15. King M, Newton M, Jackson G, et al. Epileptology of the first-seizure presentation: a clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet 1998; 352: 1007-1011.
- 16. Coory M, Walsh W. Rates of percutaneous coronary interventions and bypass surgery after acute myocardial infarction in Indigenous patients. Med J Aust 2005; 182: 507-512. <MJA full text>
- 17. Couzos S. PBS medications — improving access for Aboriginal and Torres Strait Islander peoples. Aust Fam Physician 2005; 34: 841-844.
- 18. Shamansky S, Glaser G. Socioeconomic characteristics of childhood seizure disorders in the New Haven area: an epidemiologic study. Epilepsia 1979; 20: 457-474.
- 19. Heaney D, MacDonald B, Everitt A, et al. Socioeconomic variation in incidence of epilepsy: prospective community based study in south east England. BMJ 2002; 325: 1013-1016.





Abstract
Objective: To compare patterns of epilepsy in Indigenous and non-Indigenous people presenting to hospital.
Study design: Retrospective cross-sectional survey of individuals admitted to hospital with a diagnosis of epilepsy (1 January 2001 – 31 December 2004); presenting to the emergency department with a seizure (2004); or presenting to the epilepsy clinic (1 September 2002 – 31 March 2005).
Setting: Cairns Base Hospital, the major referral centre for Far North Queensland, including Cape York and the Torres Strait, with a population of 230 000 (13% Indigenous).
Main outcome measures: Proportion of Indigenous patients presenting for epilepsy; proportion of Indigenous and non-Indigenous groups affected by each of the main epilepsy syndromes.
Results: Of 359 patients attending the epilepsy clinic and 918 patients having electroencephalography (EEG), 11% and 13% were Indigenous, respectively (in proportion with the catchment population). However, 30% (146/486) of patients presenting to the emergency department with seizure, 31% (130/418) of inpatient admissions with epilepsy, and 44% (28/63) of patients admitted with status epilepticus were Indigenous. Indigenous patients were more likely to have an abnormal EEG result (P = 0.025), while non-Indigenous patients presenting to the clinic were more likely to be classified as non-epileptic (31% v 18%). In those with abnormal EEG, the frequency distribution of abnormalities was similar, and, in those with epilepsy, syndrome classification also showed similar frequencies. There was no significant difference in occurrence of epileptogenic abnormalities detected by imaging (13% non-Indigenous v 18% Indigenous) or in alcohol consumption (38% v 37%).
Conclusions: Indigenous Australians have similar epilepsy syndromes to the non-Indigenous population, but they present with more serious disease. This discrepancy may relate to inequitable health care utilisation due to cultural issues or geographic isolation.