Intensive care services in Western Australia are very centralised, with all public and private level II and III intensive care units (ICUs) located in the Perth metropolitan area.1 Many Indigenous Western Australians live in non-metropolitan areas, and about three-quarters of this group live at least 50 km from the nearest hospital or community health care centre.2 This pattern of habitation and critical care service means all critically ill Indigenous patients from remote WA must be transferred to metropolitan tertiary hospitals for further treatment. Indigenous Australians are over-represented in ICU admissions in the Northern Territory (28% of the population but 45% of all ICU admissions),3 but there is little information on the outcome of critically ill Indigenous Australians in other parts of Australia.
This retrospective cohort study used data from the audit database of the ICU at Royal Perth Hospital. This tertiary hospital ICU admits critically ill adult patients in all clinical specialties. The database includes age and sex of the patients, severity of illness (APACHE II) scores and associated predicted mortality, admission diagnosis and source, chronic health evaluation as defined by the APACHE II prognostic model,4 ICU and hospital length of stay, and ICU and hospital mortality. The data are collected by the duty ICU consultant and are entered by designated trained clerical staff. Twelve ICU consultants, of whom seven were involved throughout the study period, were involved in collecting data using a standardised data dictionary in the 11-year period from 1 January 1993 to 31 December 2003. During this time, a single data custodian has been responsible for ensuring data quality. The data were reviewed for internal consistency annually, and there were no patients lost to follow-up or with missing data. The data used had the patients’ names and addresses removed.
The APACHE II prognostic model measures severity of illness and predicts hospital mortality of critically ill adult patients. APACHE II scores are calculated using the patient’s age, the worst measurements of 12 physiological variables within 24 hours of ICU admission, and the chronic health status of the patient.4 The APACHE II predicted mortality of a patient is calculated using the APACHE II score and admission diagnosis. The APACHE II model has been validated and used widely for research and clinical audit purposes in many ICUs.5,6 In this study, the hospital mortality of Indigenous patients was compared with the APACHE II predicted mortality across different risk strata, with the deviation from perfect prediction assessed by the Hosmer–Lemeshow H and C χ2 statistics. Patients who were admitted to the ICU after cardiac surgery were excluded from the multivariate analysis because the published APACHE II prediction model equation was not derived from patients who had undergone coronary artery bypass graft.4
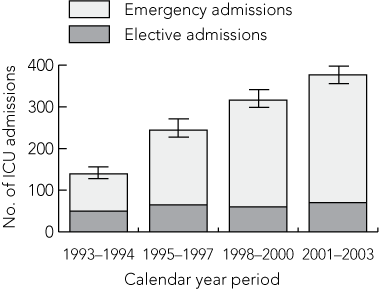
There were 16 757 ICU patients, of whom 1076 (6.4%) were identified as Indigenous Australians, in the 11 years from 1 January 1993 to 31 December 2003. Compared with non-Indigenous patients, the Indigenous patients were younger and more likely to be transferred from another hospital (Box 1). ICU admissions due to respiratory or renal failure, and the diagnoses of sepsis, pneumonia, trauma, and cardiopulmonary arrest were more common among Indigenous patients (Box 1). Indigenous people represented 3.2% of the population of WA in 2001,7 and accounted for 3.1% of all elective admissions and 9.5% of all emergency admissions. The total number of Indigenous patients admitted to the ICU each year increased over the 11-year period, mainly because of a progressive increase in emergency admissions (Box 2). Chronic liver and renal diseases were more common among Indigenous patients, and chronic cardiovascular diseases were more common among non-Indigenous patients (Box 3).
Indigenous patients admitted for elective non-cardiac surgery had a higher severity of illness, as reflected by the APACHE II predicted mortality, and higher crude ICU mortality and hospital mortality than non-Indigenous patients (Box 4).
Indigenous patients who had emergency admissions had a higher severity of illness (APACHE II scores and predicted mortality), a longer ICU stay, and higher crude ICU mortality (crude odds ratio, 1.24; 95% CI, 1.02–1.50) and hospital mortality (crude OR, 1.24; 95% CI, 1.04–1.47) than non-Indigenous patients (Box 4). The crude hospital mortality rates of both Indigenous and non-Indigenous patients were lower than predicted by the APACHE II prognostic model (Box 5).
A higher APACHE II predicted mortality, admission from the operating theatre (rather than recovery room, emergency department or other hospital), an older age, and emergency admission were significant predictors of hospital mortality in the multivariate analysis (Box 6). ICU admission during different calendar year periods, primary organ failure, ethnicity, sex, and the interaction term between admission source and ethnicity were not significant factors in determining hospital mortality in the multivariate analysis.
Intensive care treatment can be described as an “ambulance at the bottom of a cliff” with many patients admitted to the ICU only after other layers of the health care system have failed to reverse or prevent the critical illness. It is not surprising that the well described differences in the access that Indigenous patients have to primary health care, chronic disease screening and treatment, vaccination, and specialist care are reflected in a different pattern of critical illness.8-12 It is hoped that if improvements in access to comprehensive medical care occur, this will be reflected in a reduction in the severity of illness and crude mortality in ICU admissions.
The high prevalence of high acuity acute illnesses, such as sepsis, pneumonia, injuries, and cardiopulmonary arrest, among critically ill Indigenous patients is consistent with the results of other Australian studies,2,3,13-15 and very similar to data of indigenous groups from other countries.16-18 This could be due to a long delay following the onset of symptoms and reduced access to primary health care facilities or emergency departments, as most Indigenous patients reside in rural locations where primary health care facilities or staffed emergency departments are often not available.2 Furthermore, many chronic health conditions, including severe coronary heart disease, might not have been diagnosed until the patients presented to the ICU with life-threatening complications of these chronic illnesses.
There are some limitations to this study. First, this was a single centre cohort study and the results may not be generalisable to all ICUs in Australia. Royal Perth Hospital is the largest cardiothoracic surgery, cardiology, and trauma unit in WA. This may explain the large numbers of cardiac surgical and trauma admissions in this cohort and the high prevalence of chronic cardiovascular diseases among the non-Indigenous patients. Furthermore, critically ill Indigenous patients will also have been admitted to one of the other two adult tertiary public ICUs in WA, and whether the pattern of critical illness affecting these Indigenous patients is similar to our cohort remains unknown. Second, there is evidence that identification of Indigenous hospital inpatients is only 85.8% complete in WA.19 It is possible that some Indigenous patients were not identified. Third, the geographic origin of the patients was not available in the database and we cannot exclude the potential confounding effect of this factor in our results. Finally, the accuracy of the APACHE II prediction model can be affected by lead time bias when patients are referred from different sources. Many of our critically ill Indigenous patients were referred and transferred from other, usually rural or regional, hospitals. This could have created bias in the APACHE II predicted mortality, making our adjustment for hospital mortality inaccurate.20-22
Nevertheless, our findings suggest that the poor outcomes of critically ill Indigenous people are related to the high burden of chronic illness. Reduction in the burden of chronic illnesses and the incidence of high acuity acute illnesses is likely to require an improvement in primary health care, screening and early treatment of chronic diseases, vaccination, and health promotion in remote communities.8-12 There are 83 communities in WA with fewer than 50 people that are more than 50 km from a community health clinic.2 Three-quarters of these communities are not visited at least once a month by any health care professionals.2 Indigenous patients who might benefit from chronic disease screening and primary health care should have access to these health care services. Training more Indigenous health care workers for the Indigenous community has been suggested as an important step to improve the effectiveness of health care programs to Indigenous communities.23 Further improvement in the outcomes of critically ill Indigenous patients relies on a sustained improvement in access and effectiveness of preventive and primary health care services to Indigenous communities in rural Australia.12 A reduction in severity of illness and crude mortality rate in critically ill Indigenous patients may be a useful indicator of improving health outcomes in Indigenous Australians.
2 Emergency and elective intensive care unit (ICU) admissions of Indigenous patients over an 11-year period
4 Short-term outcome of critically ill Indigenous and non-Indigenous patients in elective and emergency admissions (excluding cardiac surgery)
5 Relationship between APACHE II predicted and actual hospital mortality across different risk strata (excluding cardiac surgical patients)
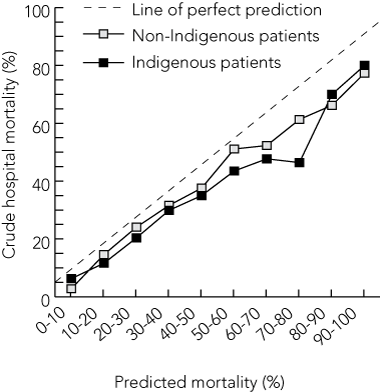
Hosmer–Lemeshow goodness of fit: H χ2 = 41.7, df = 9 (P < 0.001); C χ2 = 14.6, df = 9 (P = 0.067).
- Kwok Ming Ho1,2,3
- Judith Finn2
- Geoffrey J Dobb1,3
- Steven A R Webb1,3
- 1 Intensive Care, Royal Perth Hospital, Perth, WA.
- 2 School of Population Health, University of Western Australia, Perth, WA.
- 3 School of Medicine and Pharmacology, University of Western Australia, Perth, WA.
None identified.
- 1. Joint Faculty of Intensive Care Medicine. Minimum standards for intensive care units. Policy document IC-1. Melbourne: JFICM, 2003. Available at: http://www.jficm.anzca.edu.au/publications/policy/index.htm (accessed Apr 2006).
- 2. Statistical Information Management Committee 2004. National summary of the 2001 and 2002 jurisdictional reports against the Aboriginal and Torres Strait Islander health performance indicators. Canberra: Australian Institute of Health and Welfare, 2004. (AIHW Catalogue No. IHW 12.) Available at: http://www.aihw.gov.au/publications/index.cfm/title/10013 (accessed Apr 2006).
- 3. Stephens D. Critical illness and its impact on the aboriginal people of the top end of the Northern Territory, Australia. Anaesth Intensive Care 2003; 31: 294-299.
- 4. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818-829.
- 5. Gunning K, Rowan K. ABC of intensive care: outcome data and scoring systems. BMJ 1999; 319: 241-244.
- 6. Ho KM, Dobb GJ, Knuiman M, et al. A comparison of admission and worst 24-hour Acute Physiology and Chronic Health Evaluation II scores in predicting hospital mortality: a retrospective cohort study. Crit Care 2005; 10: R4.
- 7. Australian Bureau of Statistics. Population distribution, Indigenous Australians, 2001. Canberra: ABS, 2002. (ABS Catalogue No. 4705.0.)
- 8. Stamp KM, Duckett SJ, Fisher DA. Hospital use for potentially preventable conditions in Aboriginal and Torres Strait Islander and other Australian populations. Aust N Z J Public Health 1998; 22: 673-678.
- 9. Hoy WE, Wang Z, Baker PR, Kelly AM. Reduction in natural death and renal failure from a systematic screening and treatment program in an Australian Aboriginal community. Kidney Int Suppl 2003; 83: S66-S73.
- 10. Menzies R, McIntyre P, Beard F. Vaccine preventable diseases and vaccination coverage in Aboriginal and Torres Strait Islander people, Australia, 1999 to 2002. Commun Dis Intell 2004; 28 Suppl 1: S1-S45.
- 11. Condon JR, Barnes T, Cunningham J, Smith L. Improvements in Indigenous mortality in the Northern Territory over four decades. Aust N Z J Public Health 2004; 28: 445-451.
- 12. Hoy WE, Kondalsamy-Chennakesavan SN, Nicol JL. Clinical outcomes associated with changes in a chronic disease treatment program in an Australian Aboriginal community. Med J Aust 2005; 183: 305-309. <MJA full text>
- 13. Roche P, Krause V, Bartlett M, et al. Invasive pneumococcal disease in Australia, 2003. Commun Dis Intell 2004; 28: 441-454.
- 14. Zhao Y, Guthridge S, Magnus A, Vos T. Burden of disease and injury in Aboriginal and non-Aboriginal populations in the Northern Territory. Med J Aust 2004; 180: 498-502. <MJA full text>
- 15. McDonald SP, Russ GR. Burden of end-stage renal disease among indigenous peoples in Australia and New Zealand. Kidney Int Suppl 2003; 83: S123-S127.
- 16. Caron NR. Getting to the root of trauma in Canada’s Aboriginal population. CMAJ 2005; 172: 1023-1024.
- 17. Rutland-Brown W, Wallace LJ, Faul MD, Langlois JA. Traumatic brain injury hospitalizations among American Indians/Alaska Natives. J Head Trauma Rehabil 2005; 20: 205-214.
- 18. Davidson M, Parkinson AJ, Bulkow LR, et al. The epidemiology of invasive pneumococcal disease in Alaska, 1986–1990 — ethnic differences and opportunities for prevention. J Infect Dis 1994; 170: 368-376.
- 19. Young M. Assessing the quality of identification of Aboriginal and Torres Strait Islander people in Western Australia hospital data, 2000. Health Information Centre Occasional Paper 13. Perth: Health Department of Western Australia, 2001.
- 20. Cowen JS, Kelley MA. Errors and bias in using predictive scoring systems. Crit Care Clin 1994; 10: 53-72.
- 21. Rapoport J, Teres D, Lemeshow S, Harris D. Timing of intensive care unit admission in relation to ICU outcome. Crit Care Med 1990; 18: 1231-1235.
- 22. Combes A, Luyt CE, Trouillet JL, et al. Adverse effect on a referral intensive care unit’s performance of accepting patients transferred from another intensive care unit. Crit Care Med 2005; 33: 705-710.
- 23. Paradies YC. Affirmative action and equity in Aboriginal and Torres Strait Islander Health. Med J Aust 2005; 183: 269-270.





Abstract
Objective: To investigate the short-term outcome of critically ill Indigenous patients.
Design and participants: Retrospective cohort study using de-identified audit data from a tertiary intensive care unit (ICU) in Western Australia for the 11-year period 1 January 1993 to 31 December 2003.
Main outcome measures: Hospital mortality (crude, and adjusted for severity of illness).
Results: Of 16 757 ICU patients, 1076 (6.4%) were identified as Indigenous. The Indigenous patients were younger and more commonly had chronic liver and renal diseases. Indigenous people represented 3.2% of the population of Western Australia in 2001, but represented 3.1% and 9.5% of all elective and emergency ICU admissions, respectively. Diagnoses of sepsis, pneumonia, trauma, and cardiopulmonary arrest were common among critically ill Indigenous patients. Following emergency admission, the crude hospital mortality for Indigenous patients was higher (22.7% v 19.2%; crude odds ratio, 1.24; 95% CI, 1.04–1.47) than for non-Indigenous patients. The crude hospital mortality of critically ill Indigenous patients was lower than that predicted by the APACHE II prognostic model and was similar to that of non-Indigenous patients after adjusting for severity of illness and chronic health status.
Conclusions: The pattern of critical illness affecting Indigenous Australians in Western Australia was different from that affecting non-Indigenous patients. The crude hospital mortality was high, but similar to that of non-Indigenous Australians after adjusting for severity of illness and chronic health status.