Transmission of human immunodeficiency virus (HIV),1,2 hepatitis B virus (HBV)2 and hepatitis C virus (HCV)3,4 has been documented in correctional settings. Transmission is related to the increased prevalence of these agents in the prison population, high-risk injecting and sexual behaviour, and the limited availability of prevention methods.5,6 Post-exposure prophylaxis (PEP) against HIV has been recommended in New South Wales for non-occupational exposures to HIV since 1998.7
The aim of our study was to determine if transmission of HIV, HBV and HCV occurred after episodes of needle-sharing with two inmates with HIV infection in Australian prisons in November 2000, and to evaluate the use of HIV PEP in this setting.
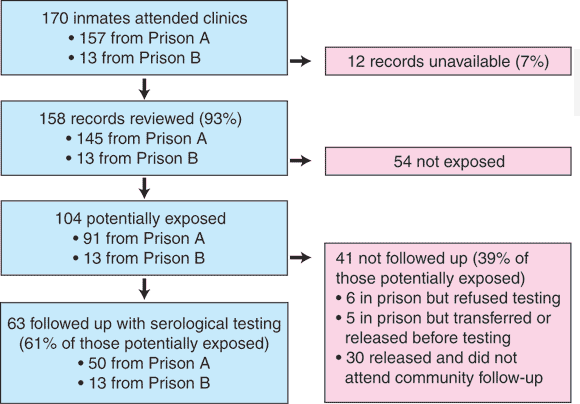
One hundred and seventy inmates attended the clinics in response to the invitation. Participants at each stage of the study are shown in Box 1. Medical records of 158 (93%) were reviewed. On the basis of information on sharing in these records, 104 inmates (66% of those reviewed) were assessed as potentially exposed, comprising 91 of the 157 inmates who attended the clinic in prison A (58%) and all 13 inmates who attended in prison B.
Baseline serological results were available for all 104 potentially exposed inmates (Box 2). Seventy-four were still in prison when their medical records were reviewed, and follow-up serological testing was conducted in 63 (61% of those potentially exposed) (Box 1).
Our study demonstrates four HCV seroconversions most likely related to high-risk needle- and syringe-sharing in prison, and outlines the first documented use of HIV PEP in the prison setting anywhere in the world. A minority of those prescribed PEP completed the course.
Only a small proportion of the inmates in either prison were suscept-ible to HBV or HCV at baseline, but all were susceptible to HIV. Our findings are consistent with the higher probability of transmitting HCV compared with HIV through sharing needles and syringes.8 Nevertheless, it is possible that HIV seroconversions did occur in inmates who were lost to follow-up. Treatment of HBV-susceptible inmates with HBV vaccination or immunoglobulin after the potentially high-risk exposure may have prevented any HBV seroconversions in those followed up.
It is difficult to be certain that each new HCV infection was related to the documented exposure. Seroconverter 1 was probably infected in prison B in November 2000, as he was a new injecting drug user and reported multiple exposures in the prison. The other HCV-infected patients all reported ongoing injecting drug use in prison and may have acquired their HCV infection after the risk period.
Index patient A did not identify contacts, meaning that all inmates who reported needle or syringe sharing in prison A were screened. This risk assessment process is likely to have overestimated the number of inmates truly exposed in prison A compared with prison B, where the cohort was smaller, and the pattern of sharing needles and syringes was more easily determined.
Inmates were initially provided with a four-week course of PEP, but it became evident that some were trading the PEP for other commodities. As a result, prison health staff started administering daily doses of PEP to inmates at the prison clinic. This method, while avoiding misuse of PEP, may have influenced discontinuation with therapy, given the frequent and unpredictable movement of inmates between prisons, courts and the community.
While guidelines indicate that individuals prescribed PEP should be followed up for six months to confirm serological status,7 this was difficult to arrange in the prison environment. A quarter of inmates who started PEP completed it. Completion is generally much better in the community.9
We have documented hepatitis C transmission in the prison setting, probably related to sharing of injecting equipment. Possible prevention measures that might be implemented include needle and syringe exchange programs, which are the community standard. While HIV PEP may be administered in the prison setting, special consideration of prison circumstances is necessary to ensure accurate risk assessment, consideration of ongoing risk behaviours, prompt initiation of therapy, good compliance and adequate follow-up. Specific guidelines for the use of PEP in prisons should be developed by correctional health services to improve the administration of PEP in the prison setting.
2: Baseline serological results of inmates potentially exposed to HIV and hepatitis B and C virus infection
Serological result |
Prison A (n = 91) |
Prison B (n = 13) |
|||||||||
HIV |
|
|
|||||||||
Negative |
91 (100%) |
13 (100%) |
|||||||||
Hepatitis B virus |
|
|
|||||||||
Non-immune* |
25 (27%) |
4 (31%) |
|||||||||
Immune or previously infected† |
63 (69%) |
9 (69%) |
|||||||||
Unknown |
3 (3%) |
0 |
|||||||||
Hepatitis C virus |
|
|
|||||||||
Negative‡ |
24 (26%) |
5 (38%) |
|||||||||
Positive§ |
67 (74%) |
8 (62%) |
|||||||||
* Hepatitis B non-immune: negative for antibodies to hepatitis B virus surface and core antigens (anti-HBs and anti-HBc, respectively). † Hepatitis B immune or previously infected: positive for anti-HBs and/or anti-HBc; 36 of this group (50%) were immune (anti-HBs-positive) ‡ Hepatitis C negative: negative for antibodies to hepatitis C virus (anti-HCV). § Hepatitis C positive: anti-HCV-positive; HCV RNA testing was not performed. |
|||||||||||
3: Characteristics of four inmates who seroconverted to hepatitis C virus (HCV) after sharing needles and syringes in November 2000 in two Australian prisons
|
|
Months to positive* |
Period of incarcer- ation |
Risk factors |
Clinical history after November 2000 |
||||||
Case (prison) |
Anti-HCV results |
November 2000 |
Ongoing |
||||||||
1 (B) |
Negative: 11 Dec 00 Positive: 15 Mar 01 |
3 |
Mar 00 –
|
First time IDU in Oct 00 in prison Shared needle and syringe without routine cleaning with Seroconverters 2 and 3, and with another anti-HCV-positive inmate (not directly with the index patient) |
None |
No seroconversion illness |
|||||
2 (B) |
Negative: 12 Dec 00 Positive: 9 May 01 |
5 |
Feb 00 –
|
Frequent IDU Shared needle and syringe with Seroconverters 1 and 3 and another anti-HCV-positive inmate (not directly with the index patient) |
Ongoing frequent shared IDU Unprotected sex |
No seroconversion illness |
|||||
3 (B) |
Negative: 12 Dec 00, 30 Mar 01 Positive: 29 Aug 01 |
5 |
Nov 00 –
|
Shared needle and syringe with Seroconverters 1 and 2, and with three other anti-HCV-positive inmates (not directly with the index patient) |
Ongoing IDU |
Non-specific recurrent illness with rash on hands and feet, malaise, arthralgia (Jul–Sep 01) Mild systemic illness (Oct 01) (anti-HIV-negative in Oct 02) |
|||||
4 (A) |
Negative: 25 Nov 00 Positive: 13 Dec 01 |
13 |
Nov 00 –
|
IDU |
IDU with sharing in prison IDU without sharing in community |
No seroconversion illness |
|||||
IDU = injecting drug use. Anti-HCV = antibodies to hepatitis C virus. * Months between last anti-HCV negative result and first anti-HCV positive result. |
|||||||||||
Received 30 October 2002, accepted 23 January 2003
- Belinda G O'Sullivan1
- Michael H Levy2
- Sharon G Barton3
- Kate A Dolan4
- John M Kaldor5
- Andrew E Grulich6
- Jeffrey J Post7
- Dominic E Dwyer8
- New South Wales Department of Health, Sydney, NSW.
- 1 New South Wales Corrections Health Service, Sydney, NSW.
- 2 University of New South Wales, Sydney, NSW.
- 3 Albion Street Centre and Department of Infectious Diseases, Prince of Wales Hospital, Sydney, NSW.
- 4 Institute of Clinical Pathology and Medical Research, Westmead Hospital, Sydney, NSW.
We thank the Department of Corrective Services and Corrections Health Service, particularly the public health nurses who assisted with follow-up of inmates. We also thank Associate Professor Andrew Lloyd, who chaired the collaborative research group, as well as Ms Evelyn Crewe and Dr Jean Downie (Institute of Clinical Pathology and Medical Research, Westmead Hospital, Sydney, NSW) for serological testing. This study was carried out as part of the New South Wales Public Health Officer Training Program, which is funded by the New South Wales Department of Health. The National Centre in HIV Epidemiology and Clinical Research is funded by the Commonwealth Department of Health and Ageing.
None identified.
- 1. Dolan K, Wodak A. HIV transmission in a prison system in an Australian State. Med J Aust 1999; 171: 14-17. <MJA full text>
- 2. Taylor A, Goldberg D, Emslie J, et al. Outbreak of HIV infection in a Scottish prison. BMJ 1995; 310: 289-292.
- 3. Haber P, Parsons S, Haper S, et al. Transmission of hepatitis C within Australian prisons. Med J Aust 1999; 171: 31-33. <MJA full text>
- 4. Post JJ, Dolan KA, Whybin LR, et al. Acute hepatitis C virus infection in an Australian prison inmate: tattooing as a possible transmission route. Med J Aust 2001; 174: 183-184. <MJA full text>
- 5. Butler TG, Dolan KA, Ferson MJ, et al. Hepatitis B and C in New South Wales prisons: prevalence and risk factors. Med J Aust 1997; 166: 127. <MJA full text>
- 6. National Centre in HIV Epidemiology and Clinical Research. HIV/AIDS, viral hepatitis and sexually transmissible infections in Australia annual surveillance report 2001. Sydney: National Centre in HIV Epidemiology and Clinical Research, University of New South Wales, 2001.
- 7. Management of non-occupational exposure to blood borne and sexually transmissible diseases. New South Wales Health Department Circular 99/31, 31 Mar 1999. (supersedes circular 98/106, 12 Nov 1998).
- 8. Bodsworth NJ, Robertson M, Kaldor J. Transmission of hepatitis C virus but not human immunodeficiency virus type 1 following sharing of cleaned injecting equipment. Genitourinary Med 1994; 70: 206-207.
- 9. Correll P, Smith D, Grulich A. Post exposure prophylaxis for non-occupational exposure to HIV: experience in New South Wales one year after the guidelines. NSW Public Health Bull 2000; 11: 114-117.





Abstract
Objectives: To determine whether infection with human immunodeficiency virus (HIV), hepatitis B virus (HBV) or hepatitis C virus (HCV) occurred after two potential episodes of exposure through needle- and syringe-sharing in Australian prisons, and to examine use of post-exposure prophylaxis (PEP) against HIV infection in the prison setting.
Design: Cohort study of potential contacts of two prisoners infected with HIV, HBV and HCV followed up for up to 14 months.
Setting: Two Australian prisons between November 2000 (time of exposure) and December 2001.
Participants: Two index patients (both infected with HIV and HCV; one also infectious for HBV) from two different prisons, and 104 inmates who shared needles and syringes.
Main outcome measures: Seroconversions to HIV, HBV and HCV related to the high-risk exposure and uptake and completion of HIV PEP determined from medical records of inmates.
Results: There were four seroconversions to HCV within 14 months of the potential exposure (14% of those susceptible in the cohort), but no recorded HIV or HBV seroconversions. Forty-six inmates (82% of those eligible) were offered PEP, and 34 of these (74%) elected to receive it. Only eight (24% of the 34) completed the full PEP course.
Conclusions: HCV transmission in the prison setting is related to high-risk needle- and syringe-sharing. Administering HIV PEP in the prison setting is complicated by challenging risk assessment and follow-up.