Transmission of hepatitis C within Australian prisons
Transmission of hepatitis C virus (HCV) within prisons has long been suspected but has not been satisfactorily documented. We present four cases of HCV infection occurring during periods of continuous imprisonment. Each subject was HCV seronegative on entering prison and on repeat testing after 4-52 months in prison, but subsequently became seropositive. Two subjects gave a history of injecting drug use, and the most likely means of infection in the other two subjects were lacerations from barbers shears and lacerations arising from physical assault. There is an urgent need for detailed study of the incidence of HCV infection and the modes of transmission in prisons.
Introduction
The prevalence of hepatitis C (HCV) infection in voluntary screening among entrants to Australian and North American prisons is as high as 40%.1-3 It is therefore surprising that HCV transmission within prisons has not been well documented, although it is known that a history of incarceration is an independent risk factor for HCV seroconversion4 and uninfected prisoners are at high risk of seroconversion by the time of a second prison entry.1,3 These observations provide only indirect evidence of transmission within prisons, as infection could have occurred either during the first period of imprisonment or outside prison between release and re-incarceration. This report describes the first published series of well-documented cases of transmission of HCV within a prison.
The Research Ethics Committee of the Corrections Health Service of New South Wales approved reporting of these cases.
Clinical records
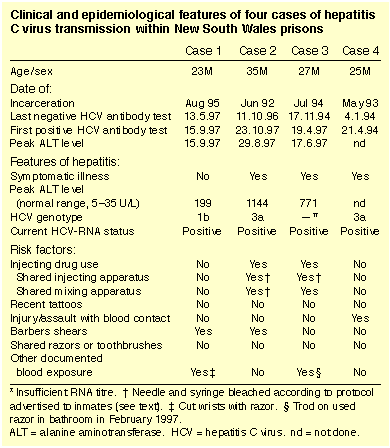
Between April 1994 and October 1997, four male prisoners presented with HCV infection appearing a minimum of 11 months after entry to prison (Table). All had had negative HCV antibody tests on entry to prison and again after 4-52 months of continuous incarceration (anti-HCV ELISA version III, Murex Diagnostics, Kyalamani, South Africa; confirmation by Innotest HCV Ab III assay, Innogenetics, Zwijnaarde, Belgium). All bore tattoos, but all four stated that no tattoos had been applied within two years of the last negative HCV antibody test. None had received blood products, or undergone medical or dental procedures, including vaccination, in the year before acquisition of HCV. All four denied any body piercings within two years of the last negative HCV antibody test, or sexual intercourse with another person since entering prison.
Case 1
A 23-year-old inmate requested an HCV antibody test after receiving a scalp laceration during a close-shave haircut (performed with electric shears without a plastic guard) in June 1997. There was minimal bleeding and the wound did not require suturing. The patient denied having injected drugs at any time, but admitted to smoking marijuana and snorting cocaine in the past. Indeed, he regarded any injection with abhorrence and feared bloodborne infection. As a result, he had requested HCV antibody tests after numerous low risk events in prison and was repeatedly seronegative. After this latest incident, the patient again requested serological tests for HCV, and seroconversion was documented six weeks later. The patient stated that several of the four preceding inmates on whom the barbers shears were used on the same day were HCV antibody positive and had also received minor lacerations during their haircuts. He also claimed that the electric shears were not disinfected in any way between uses. No further details are available of the barber's list for that day, as no permanent records of these lists are kept. Sterilisation of shears before reuse was not normal practice at the time. One of the other inmates on whom the shears were used was confirmed to be positive for HCV antibody and HCV-RNA by polymerase chain reaction (PCR) analysis (Amplicor HCV detection kit, Roche Diagnostic Systems, Branchburg, NJ, USA).
Case 2
A 35-year-old inmate suffering from lassitude and anorexia presented for examination. He had started using intravenous drugs for the first time after being in prison for several years. For a period of six months, he had injected two to three times per week, frequently sharing needles with known HCV-seropositive inmates, but routinely sterilising the injecting apparatus using a recommended bleaching protocol with 5.25% hypochlorite solution.4 Six weeks after ending drug use, he developed the symptoms described, which, in association with raised alanine aminotransferase (ALT) levels, were consistent with viral hepatitis. HCV seroconversion was found to have taken place since his last negative test one year previously.
Case 3
A 27-year-old inmate presenting with lassitude was found to have abnormal liver function tests typical of acute viral hepatitis. The patient was an infrequent injecting drug user who had continued his habit intermittently during imprisonment. As he was aware of the risk of transmission of bloodborne pathogens, he used a bleached injecting apparatus for each episode, but shared the mixing spoon in which the drug suspension was prepared. HCV seroconversion was confirmed.
Case 4
A 25-year-old inmate presented for examination after being involved in a physical assault with another inmate, during which blood-to-blood contact occurred from abrasions and lacerations. He had sustained no serious injury. The other inmate was a known HCV-positive injecting drug user who later left prison and was lost to follow-up. Blood taken soon after the assault was negative for HCV antibodies, but seroconversion, associated with mild symptoms of hepatitis, was documented three months later. The patient denied injecting drug use.
Discussion
These cases provide strong evidence that transmission of HCV occurs in prison. Infection during injecting drug use is likely to be a leading mode of HCV transmission in prisoners. Inmates report that injecting apparatus is scarce in the prison system, whereas heroin is readily obtained. These circumstances favour repeated use of a limited number of needles and syringes by many prisoners. The recommended bleach cleansing of the injecting equipment appears ineffective, as our report corroborates previously documented transmission of HCV, but not HIV, after sharing of cleaned injecting apparatus.5
In two of the cases, HCV may have been transmitted by means unrelated to injecting drug use. The high prevalence of HCV among those entering prison, together with the strong likelihood of blood-to-blood contact in the prison environment, may increase the chance of HCV transmission by barbers shears, during physical assault or by other mechanisms.6 However, a limitation of our study is the reliance on self-reporting of risk factors by prison inmates. Inmates' self-reporting is relatively accurate if their status is unlikely to be affected by the content of the report, but may be biased if they perceive that harm or benefit may result.7
Our study provides the strongest evidence to date that transmission of HCV infection occurs within prisons. Two cases of HCV seroconversion among prisoners in Maryland (USA) have been reported:2 of 164 prisoners who tested negative for HCV on entry to prison, two tested positive 18 months later. However, there was not unequivocal evidence that HCV transmission occurred within prison, as the initial negative HCV test may have been carried out during the window phase between infection and seroconversion if viral transmission occurred outside prison shortly before incarceration. In our study, all four subjects were seronegative for HCV after 4-52 months' continuous imprisonment, and remained in continuous full-time custody until seroconversion was documented (Table).
Approximately 10 500 imprisonments occur annually in New South Wales, which has a population of 6 300 000. At any one time, the NSW prison population is about 6000. The average sentence is seven months, and there are more than 25 000 transfers between prisons each year. Thus, the prison population has a high turnover and is not isolated from the general community. The extent of HCV transmission may be significant because of the prevalence of high-risk behaviours in prison, and the fact that some harm-reduction measures, such as needle exchange programs, are not available in Australian prisons. Thus, the prison community is a population at significant risk of HCV infection and a potentially important source of subsequent transmission of HCV into the general community.
The cases presented here probably represent only a small fraction of inmates acquiring new HCV infection in prison. Firstly, our cases were detected clinically (whereas most primary HCV infections are subclinical) and, secondly, our study did not attempt a systematic search for HCV transmission among inmates. Moreover, there are rare occurrences of HCV infection without detectable HCV antibodies, and such cases depend on HCV-PCR testing for diagnosis.8
This report confirms that HCV is currently being transmitted within NSW prisons. The circumstances for acquisition of serious bloodborne infections during imprisonment should be identified and opportunities for transmission minimised where possible. Accordingly, detailed studies of the incidence of and risk factors for HCV transmission within prisons are urgently needed, followed by development and implementation of control measures.
References
- Crofts N, Stewart T, Hearne P, et al. Spread of bloodborne viruses among Australian prison entrants. BMJ 1995; 310: 285-288.
- Vlahov D, Nelson KE, Quinn TC, Kendig N. Prevalence and incidence of hepatitis C infection among male prison inmates in Maryland. Eur J Epidemiol 1993; 9: 566-569.
- Butler TG, Dolan KA, Ferson MJ, et al. Hepatitis B and C in New South Wales prisons: prevalence and risk factors. Med J Aust 1997; 166: 127-130.
- van Beek I, Dwyer R, Dore GJ, et al. Infection with HIV and hepatitis C virus among injecting drug users in a prevention setting: retrospective cohort study. BMJ 1998, 317: 433-437.
- Bodsworth NJ, Robertson M, Kaldor J. Transmission of hepatitis C but not human immunodeficiency virus type 1 following sharing of injecting equipment. Genitourin Med 1994; 70: 206-207.
- Gill ON, Noone A, Heptonstall J. Imprisonment, injecting drug use, and bloodborne viruses: a threat of transmission but an opportunity for prevention. BMJ 1995; 310: 275-276.
- Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend 1998; 51: 253-263.
- Gretch DR. Diagnostic tests for hepatitis C. Hepatology 1997; 26 Suppl 1: 43S-47S.
Authors' details
Drug and Alcohol Services, Royal Prince Alfred Hospital, Sydney, NSW.
Paul S Haber, MD, FRACP, Staff Specialist.
Public Health Nursing Unit, Corrections Health Service, Sydney,
NSW.
Sandra J Parsons, RN, Clinical Nurse Consultant;
Susan E Harper, RN, Public Health Nurse.
Virology Division, Department of Microbiology, South Eastern
Sydney Area Laboratory Services, Sydney, NSW.
William D Rawlinson, PhD, FRACP, FRCPA, Division
Head.
Peter A White, PhD, Research Fellow.
School of Pathology, University of New South Wales, Sydney, NSW.
Andrew R Lloyd, MD, FRACP, Associate Professor,
Inflammation Research Unit.
- Paul S Haber
- Sandra J Parsons
- Susan E Harper
- Peter A White
- William D Rawlinson
- Andrew R Lloyd
- 1.
- Crofts N, Stewart T, Hearne P, et al. Spread of bloodborne virusesamong Australian prison entrants. BMJ 1995; 310: 285-288.
- 2.
- Vlahov D, Nelson KE, Quinn TC, Kendig N. Prevalence and incidence ofhepatitis C infection among male prison inmates in Maryland. Eur JEpidemiol 1993; 9: 566-569.
- 3.
- Butler TG, Dolan KA, Ferson MJ, et al. Hepatitis B and C in New SouthWales prisons: prevalence and risk factors. Med J Aust 1997;166: 127-130.
- 4.
- van Beek I, Dwyer R, Dore GJ, et al. Infection with HIV and hepatitis Cvirus among injecting drug users in a prevention setting:retrospective cohort study. BMJ 1998, 317: 433-437.
- 5.
- Bodsworth NJ, Robertson M, Kaldor J. Transmission of hepatitis Cbut not human immunodeficiency virus type 1 following sharing ofinjecting equipment. Genitourin Med 1994; 70: 206-207.
- 6.
- Gill ON, Noone A, Heptonstall J. Imprisonment, injecting drug use,and bloodborne viruses: a threat of transmission but an opportunityfor prevention. BMJ 1995; 310: 275-276.
- 7.
- Darke S. Self-report among injecting drug users: a review. DrugAlcohol Depend 1998; 51: 253-263.
- 8.
- Gretch DR. Diagnostic tests for hepatitis C. Hepatology1997; 26 Suppl 1: 43S-47S.