Randomised controlled trials (RCTs) are seen as the evidence "gold standard" for the effectiveness of clinical interventions. However, as a method of evaluating complex interventions in community-based settings they have significant limitations. Because of the poor uptake in Australia of secondary prevention for alcohol misuse among Indigenous people,1 we set out to trial a brief intervention (involving motivational interviewing of individuals not presenting primarily with an alcohol problem2) in this population. This intervention has been subjected to a rigorous multinational trial under the auspices of the World Health Organization (WHO)3 and found to help patients reduce their risk of alcohol-related harm.4,5 However, there were too few Indigenous people in the Australian arm of the WHO trial to provide evidence of the effectiveness of the intervention in this population. Here we report on an attempt to address this gap through implementation of a trial in an urban Aboriginal Medical Service.
This study was a joint community–university partnership which involved Aboriginal Medical Service (AMS) Board approval; joint application to the National Health and Medical Research Council (NHMRC) to develop, pilot and implement the trial; AMS management of grant funds and employment of the project officer; joint development of the study protocol; and AMS control over all aspects of implementation. The three key factors underpinning the partnership's success were that
-
the AMS was committed to trying a new approach to alcohol misuse and was willing to consider formal research processes;
-
the AMS employed the project officer, who immersed herself in the daily life of the clinic, allowing for ongoing debate and negotiation with staff at all levels; and
-
the development of a Memorandum of Understanding in which the partners agreed details relating to receipt, management and allocation of grant funds, study design, data collection, storage and access, and community feedback and publication.
Ethical approval for this study was obtained from the Australian National University Human Research Ethics Committee.
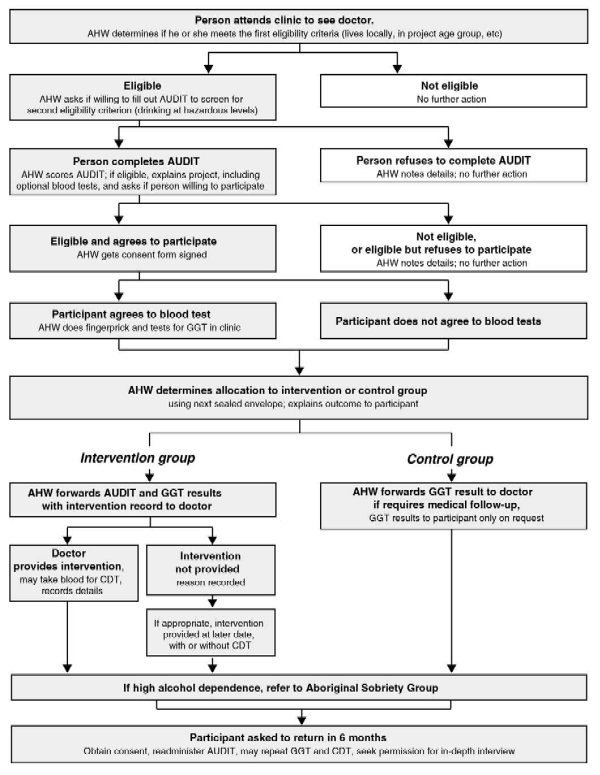
The primary objective of the study was to determine the acceptability (including cultural appropriateness) and effectiveness of brief intervention in at-risk attendees of an urban AMS. We were also interested in determining whether training in brief intervention helped providers broach and address the difficult topic of alcohol use with Aboriginal patients. The project was based in the AMS's two busiest clinics. The design included using the internationally validated 10-question screening tool known as the Alcohol Use Disorders Identification Test (AUDIT) to screen clinic attendees, random allocation of hazardous drinkers to either brief intervention or usual care, an on-the-spot fingerprick blood test for γ-glutamyltransferase (GGT), a blood sample drawn for laboratory testing for carbohydrate-deficient transferrin (CDT), and follow-up in six months for repeat AUDIT and blood tests. Those determined eligible by AUDIT screening were given a more detailed explanation of the project, including the meaning and implications of random allocation to control and intervention groups, and asked to sign the consent form, which had separate spaces for consent to the blood tests. Because of sensitivity about this issue among Indigenous people, it was agreed that patients should be able to participate without having either blood test.
We estimated we needed around 400 participants (200 intervention, 200 control) to complete follow-up. This estimate was based on the conservative assumptions that by the end of the trial between 50% and 95% of control-group clients would still be drinking at hazardous levels (AUDIT score of 6 or more for women, 7 or more for men), and that there would be a 20% difference between the intervention and control groups (at a significance level of 1% and power of 90%). Thus, allowing for 20% attrition, we needed to recruit around 500 people. About 2500 clients had attended the AMS in 1996, of whom around half were in the study age range of 18–65 years. AMS staff estimated that around 40% of these might be hazardous drinkers, leaving an estimated 500 clients in a year eligible to take part. This meant we had a margin for refusal and loss to follow-up of only 20%, and so we anticipated possibly having to extend recruitment beyond the one year planned.
We recruited a project officer to work on site, and the whole team worked with AMS staff to develop a study protocol. Having two clinics, six general practitioners (GPs) to train in brief intervention, many Aboriginal health workers and nurses involved in screening and blood testing and fairly complex testing and laboratory pick-up logistics required extensive planning and coordination. Significant effort was also made to generate interest in and commitment to the project among the staff.
The project protocol was revised several times before it was felt to be adequate. However, it remained extremely complicated (see Box 1), particularly in the context of a busy health clinic. It involved Aboriginal health workers approaching clients, administering the AUDIT, scoring the responses to determine whether clients were drinking at hazardous or harmful levels, tagging the client's file, seeking participation, obtaining informed consent, undertaking the fingerprick GGT test, and completing the randomisation procedures. GPs were responsible for conducting the brief intervention, collecting some additional information, and taking the blood sample for CDT testing. In addition, the project officer had to develop methods for determining potential eligibility for the project and keeping track of clients and their data as they moved through the project, the latter made more complicated by the number of staff involved.
We began a pilot of the study protocol in November 1997. During the first week, 40 people were asked by the Aboriginal health workers if they would complete the AUDIT. Of these, 35 declined. Of the five who agreed, all had scores indicating "hazardous" (or the more extreme "harmful") use, and all agreed to take part in the trial.
The low participation rate was attributed to a number of factors, but primarily the reluctance of patients to answer questions about their use of alcohol, particularly when asked by other Aboriginal people whom they knew. This reluctance also extended to non-Aboriginal staff with whom patients had ongoing contact. Aboriginal health workers also seemed reluctant to ask people to complete the AUDIT.
To overcome these barriers, a revised protocol was developed and a second pilot carried out. This time, the project officer, who was non-Aboriginal and not known to patients, was responsible for the screening, consent and random allocation processes. She sat in the AMS reception area and asked people if they were willing to fill out the AUDIT. Of the 12 people asked in this way, five agreed to fill in the AUDIT, of whom four agreed to take part in the project. While this resulted in slightly improved recruitment, it lessened the integration of the intervention into clinical processes. Difficulty in assessing who met the pre-screening eligibility criterion (come to see a doctor) and lack of privacy in the reception area also made this method of screening inefficient and more disruptive.
After 15 working days, 53 attendees had been approached, but only 10 had agreed to complete the AUDIT. All 10 were scored as drinking above safe levels and nine had agreed to participate in the study. Based on our recruitment estimates we needed to have recruited at least 30 participants — two per day — by this time, but had recruited less than one per day.
We suspended the project and early in the new year undertook a round of meetings and interviews with all the relevant staff to try to identify what the problems were. The key points to emerge were:
-
project processes did not fit well with clinic processes and the project was a "hassle";
-
alternative project processes preferred by staff did not fit the study protocol;
-
patients were embarrassed or resentful about being approached by Aboriginal health workers about their drinking, and did not want to discuss it with people who often already knew about their drinking;
-
the Aboriginal health workers felt uncomfortable approaching the patients about drinking (especially those who were older, known to them socially, or members of their extended families) and were uncomfortable administering the AUDIT, which was seen as too long and too intrusive;
-
random allocation was felt by Aboriginal health workers to be unethical, on the one hand, and like "telling people what to do", on the other;
-
Aboriginal health workers felt under pressure to recruit people;
-
there was a perception that there was no incentive for patients to be in the project; and
-
there was a strong preference among the Aboriginal health workers and nurses for the GPs to take over most of the responsibilities of approaching the issue of drinking with clients, administering the AUDIT, obtaining consent, and random allocation, as well as conducting the brief intervention.
The randomised design was subsequently abandoned in favour of a two-part "demonstration project" with a specific focus on the acceptability and cultural appropriateness of the intervention, and on the impact of training on the providers' ability to address alcohol-related issues with clients. Under Part A, brief intervention was offered by the GPs to any patient who might benefit as part of standard clinical practice. This was predicated on the demonstrated effectiveness of the intervention in other settings. Part B comprised a study of a subset of patients who received the intervention (ie, consent was sought for participation in an effectiveness study). Initial screening by the Aboriginal health workers was reduced to two questions already on the clinic's client induction form, while responsibility for other study processes was transferred to the GPs, including screening (where this had not already been done), and administering the AUDIT. The AUDIT was now to be used as the baseline assessment rather than the screening tool.
The project recommenced on April 15, 1998. Over the next six months, about 900 individuals in the study age range had a consultation with a GP and the great majority of these were screened using the two questions (it was not possible to determine the exact numbers). Of the 900 attendees, only 64 were identified as drinking at hazardous levels. Of these 64, 25 received the intervention and 16 of these agreed to take part in the effectiveness study. Instead of the original target of two people per week we had recruited only one per fortnight. After much debate the project was brought to an end and we wrote to the NHMRC to terminate our funding.
Clinic, patient, Aboriginal health worker and GP factors, interacting with study design factors, all contributed to our inability to implement the trial as designed. Two different sets of clinic processes; the inevitable complexity of the study protocol; problems with the screening technique; patient reluctance to talk about alcohol consumption; sensitivity of the staff about broaching the subject; staff attitudes to random allocation (also reported by others6); GP reluctance or inability to follow through with eligible attendees because of discomfort, patient ill-health or time constraints; and patient reluctance to be involved in research — all contributed to the study's non-viability. In addition, we may have overestimated the numbers likely to be eligible. Many of those screened appeared to be non-drinkers, perhaps partially explained by the fact that the health service provided nearly twice as many consultations for women as it did for men. Males were particularly under-represented in the 16–44-years age range. In addition, 27% of all consultations were for people aged 0–17 years. Only 8% of consultations were for those aged 16–25 years. Thus, people in the age groups most likely to drink at hazardous levels were under-represented among AMS attendees. Similarly low recruitment to a GP brief intervention study has been described elsewhere.7
Received 20 July 2001, accepted 12 December 2001
- 1. Brady M. Broadening the base of interventions for Aboriginal people with alcohol problems. Technical Report No 29. Sydney: National Drug and Alcohol Research Centre, 1995.
- 2. Saunders J, Lee N. Opportunistic brief interventions. Medicine 1999; 27: 22-33.
- 3. Babor T, Grant M, editors. Project on identification and managements of alcohol-related problems. Report on phase II: a randomized clinical trial of brief interventions in primary care. Geneva: World Health Organization, 1992.
- 4. Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ 1988; 297: 663-668.
- 5. Bien T, Miller W, Tonigan J. Brief interventions for alcohol problems: a review. Addiction 1993; 89: 315-336.
- 6. Fairhurst K, Dowrick C. Problems with recruitment in a randomized controlled trial of counselling in general practice: causes and implications. J Health Serv Res Policy 1996; 1: 77-80.
- 7. Richmond R, G-Novak K, Kehoe L, et al. Effect of training on general practitioners' use of a brief intervention for excessive drinkers. Aust N Z J Public Health 1998; 22: 206-209.





This research was funded by the National Health and Medical Research Council. We would like to especially thank Board Chair Basil Sumner, and the staff of Nunkuwarrin Yunti, including Dr John Agzarian, Ms Bernadette Blackman, Dr Diwa Cabaron, Mr Ross Cameron, Dr Nicola Chynoweth, Ms Christine Clarke, Ms Molly Collins, Ms Julie Coulthard, Mrs Frances Csorba, Ms Vicki Holmes, Ms Julie McAlistair, Dr Damien Mead, Dr Srimal Nawana, Mr Lindsay Osborn, Dr Veda Rengasamy, Ms May Turner and Ms Geraldine Wilson, who contributed to the project in various ways. Others who contributed are staff of the National Centre for Education and Training in the Addictions (NCETA); and Dr John Whitfield, Royal Prince Alfred Hospital.
None declared.