|
Research
Why do preterm infants die in the 1990s?
Lex W Doyle, Sheryle Rogerson, Shu-Ling Chuang, Matthew James,
Ellen
D Bowman and Peter G Davis
MJA 1999; 170: 528-532
Abstract -
Introduction -
Methods -
Results -
Discussion -
References -
Authors' details
-
-
Articles on similar material
|
Abstract |
Objectives: To describe the mortality rate for
preterm infants (born 23-36 completed weeks' gestational age) and to
determine the causes of death, focusing on avoidable causes.
Design and setting: Prospective cohort study of preterm
infants born at Royal Women's Hospital, Melbourne (a tertiary
referral hospital with a neonatal intensive care unit and a special
care nursery) from January 1994 to December 1996.
Subjects: 2475 consecutive liveborn infants with
gestational ages from 23 to 36 weeks.
Main outcome measures: Mortality rate during the primary
hospitalisation, and causes of death.
Results: The total mortality rate was 4.8% (118/2475).
The mortality rate declined with increasing maturity. The decrease
in mortality was rapid between 23 and 28 weeks' gestational age, from
64.5% at 23 weeks to 4.0% at 28 weeks, then slower, falling to 0.4% at 36
weeks. Fifty of the 118 infants who died had lethal congenital
anomalies. Lethal anomalies accounted for three-quarters of deaths
in infants aged 28-36 weeks. The mortality rate in infants free of
lethal anomalies was 2.8% (68/2425) and only 0.2% (4/1759) for
infants aged 32-36 weeks. In the 68 infants without lethal anomalies
who died, few obvious preventable causes were identified.
Conclusions: Mortality rates fell rapidly between 23 and
28 weeks' gestational age. Survival rates for preterm infants born
after 31 weeks' gestational age approached the survival rates of term
infants. Lethal congenital anomalies were the most common cause of
death; preventable causes of death were rare.
|
| | Introduction |
The survival rate for very preterm infants (born at 23-27 weeks'
gestational age) has improved dramatically with advances in
perinatal care,1 particularly efforts to
reduce neonatal respiratory distress syndrome (hyaline membrane
disease), such as administering corticosteroids to the mother
before delivery,2 administering exogenous
surfactant to preterm infants,3 and assisted
ventilation.4 However, mortality rates
and causes of death for more mature but still preterm infants (28-36
weeks' gestational age) have received little attention, especially
since the advent of exogenous surfactant in Australia in 1991. As
hyaline membrane disease has diminished as a cause of death of preterm
infants, other causes of death, some of which may be preventable, have
assumed more prominence.
The aim of this study of preterm infants 23-36 weeks' gestational age
born in 1994-1996 in a hospital with neonatal intensive care
facilities was to describe the variation with gestational age in the
mortality rate and the causes of death, focusing on avoidable causes.
|
| |
Methods |
| |
Setting |
This was a prospective cohort study of consecutive livebirths
between 23 and 36 completed weeks of gestational age in the Royal
Women's Hospital, Melbourne, over the three years from 1 January 1994
to 31 December 1996.
| |
Data collection |
Data have been collected prospectively since 1977 on all infants
admitted to the neonatal intensive care unit (NICU), all infants of
birthweight below 1500 g, and all infants below 32 weeks' gestational
age, whether admitted to the NICU or not. Data on infants 32 to 36 weeks
were collected from the special care nursery (SCN) admissions book,
and the hospital's main computer database was checked to obtain data
on those not admitted to either the NICU or SCN.
Data collected included gestational age, birthweight, and
mortality during the primary hospitalisation, whether during the
neonatal period (first 28 days after birth) or later. Data were
included for infants transferred from our hospital to another.
Gestational age in completed weeks was assigned according to the
first antenatal ultrasound scan, or maternal dates if no ultrasound
report was available. Birthweight ratio was calculated by dividing
the infant's birthweight by the expected gender-specific median
birthweight for that gestational age.5 | |
Causes of death |
For infants who died, the major causes of death were determined after
the regular monthly clinicopathological conference at which the
deaths of all livebirths within the hospital were discussed, and
which was chaired by one of the authors ( LW D), who retained all records
from the meeting. Causes of death included lethal congenital
anomalies, complications of prematurity, perinatal asphyxia, or
sepsis (Box 1).
| |
Preventable causes of death | |
For each infant who died, avoidable factors included whether
antenatal corticosteroids or surfactant had been given to infants
who died from respiratory causes, whether intramuscular vitamin K
had been given to those who died from pulmonary haemorrhage, whether
appropriate anti-infective therapies were given to those who died
from infection, and whether antenatal corticosteroids had been
given to those who died from cerebroventricular haemorrhage or
cystic periventricular leukomalacia.
| |
Data analysis |
Mortality data for preterm births at the Royal Women's Hospital were
compared with published data for livebirths in Victoria in
1994,7 1995,8 and
1996,9 and for admissions to
neonatal intensive care units in Australia and New Zealand in
1994.10 Data were edited and analysed with SPSS for Windows.11 Dichotomous
variables were contrasted by  2 analysis, and continuous
variables were compared by Mann-Whitney U test,12 as most data
were skewed. 2 analysis, and continuous
variables were compared by Mann-Whitney U test,12 as most data
were skewed.
|
| |
Results |
| |
Causes of death |
There were 2475 infants of 23-36 weeks' gestational age born during
the study period, of whom 118 died (4.8%). The mortality rate
diminished rapidly between 23 and 28 weeks' gestational age (from
64.5% at 23 weeks to 4.0% at 28 weeks), then more slowly, to reach 0.4% at
36 weeks (Box 2). The autopsy rate in the infants who died was 51.8%
(59/114), with no data on autopsies for four infants who died after
transfer to other hospitals.
Fifty infants died of lethal anomalies, all but two within 28 days of
birth (Box 3). Lethal anomalies accounted for 11 of 67 deaths (16.4%)
in infants born at 23-27 weeks' gestational age, 16 of 24 deaths
(66.7%) in infants born at 28-31 weeks' gestational age, and 23 of 27
deaths (85.2%) in those born at 32-36 weeks' gestational age.
Sixty-eight infants died who were without lethal anomalies: 2.8% of
the 2425 livebirths, but only 0.2% of infants of 32-36 weeks'
gestational age (4/1759) (Box 4). Sixty-one died within 28 days of
birth and seven (10.3%) died later during the primary
hospitalisation. In the Australian and New Zealand Neonatal
Network, 35 of 256 deaths (13.7%) of infants of less than 32 weeks'
gestational age without lethal anomalies died during the primary
hospitalisation but after 28 days of age.10 | |
Preventable causes of death | |
Respiratory problems: The mothers of 23 of the 30
infants who died from HMD or BPD had received antenatal
corticosteroids. In the remaining seven cases, the gestational ages
were 23 or 24 weeks. In six cases there was not enough time to give
corticosteroids, five mothers presenting in advanced preterm
labour and one with eclampsia. In the seventh case, the gestational
age was 23 weeks and before birth the infant was considered incapable
of surviving, yet was given full intensive care postnatally.
All 30 infants who died from HMD or BPD received exogenous surfactant
after birth.
The one infant who died from pulmonary haemorrhage without HMD
received antenatal corticosteroids and did not have respiratory
distress after birth and therefore did not re- ceive exogenous
surfactant; the fatal pulmonary haemorrhage occurred at three days
of age.
The two infants who had HMD and pulmonary haemorrhage also received
antenatal corticosteroids.
All three infants who died from pulmonary haemorrhage had received
intramuscular vitamin K at birth. None had clinical signs of heart
failure or a patent ductus arteriosus before the haemorrhage. None
had clinical indications for an echocardiogram, hence subclinical
cardiac dysfunction cannot be excluded.
Considering the 12 infants in the gestational age range 28-36 weeks
who died without lethal anomalies, six died from sepsis, two from
asphyxia, two from BPD, one from pulmonary haemorrhage, and one from
SIDS. None died acutely from HMD.
Infections: In the 22 infants who died from sepsis
without NEC, there were only two in whom the management might have been
different.
In one infant of 30 weeks' gestational age the mother ignored the
antenatal signs of sepsis for several days before presenting to
hospital and delivering a moribund infant who died at 11 hours of age
from E. coli pneumonia, despite full treatment, including
appropriate antibiotics.
In the other infant, of 33 weeks' gestation, who died of herpes
simplex, the clinical presentation was that of sepsis several days
before death, but, in the absence of other features of herpes in the
mother or infant, no antiviral therapy was given.
CVH and CPVL: Antenatal corticosteroids were given
to the mothers of all nine infants in whom CVH or CPVL were major
contributing causes of death. In addition to the five infants in whom
CVH was a major cause of death, another 12 infants who died had a grade 3
or 4 CVH that was thought not to contribute substantially to their
deaths.
| |
Comparison with regional outcomes | |
The neonatal survival rate for infants of 24-31 weeks' gestational
age at our hospital was similar to that reported in regional data from
Victoria for the same three years7-9 (Box 5). In livebirths of
less than 2500 g birthweight in Victoria in 1994-1996, 377 infants
died within 28 days; 33.4% with lethal anomalies (compared with 44.0%
in our hospital cohort;  2 = 3.7, not
significant) and 5.6% died from infections (compared with 24.8% in
our hospital; 2 = 3.7, not
significant) and 5.6% died from infections (compared with 24.8% in
our hospital;  2 = 32.9, P
< 0.0001). The neonatal mortality rate in livebirths free of
lethal malformations with birthweights greater than 2499 g was 0.45
per 1000 (81/178834) in Victoria in 1994-1996,7-9 compared with
2.27 per 1000 (4/1735) in infants of 32-36 weeks' gestational age in
our hospital. 2 = 32.9, P
< 0.0001). The neonatal mortality rate in livebirths free of
lethal malformations with birthweights greater than 2499 g was 0.45
per 1000 (81/178834) in Victoria in 1994-1996,7-9 compared with
2.27 per 1000 (4/1735) in infants of 32-36 weeks' gestational age in
our hospital.
The survival rate to hospital discharge in 1994 for infants free of
lethal malformations cared for in neonatal nurseries at 23-31 weeks'
gestational age was higher in our hospital than in reported data from
the Australian and New Zealand Neonatal Network (Box 6).
|
| |
Discussion |
Comparing our data with regional data in Australia is difficult.
States and territories provide data to the Australian Institute of
Health and Welfare (AIHW), which then produces an annual
report.13 However, the denominator
in the AIHW report is predominantly determined by birthweight;
gestational age is reported for confinements (ie, mothers), or for
births, but not for all livebirths. Regional reports from Victoria
provide data for births, including stillbirths and livebirths, by
gestational age, but in two-week intervals, and only up to 31 weeks'
gestational age.7-9 Moreover, the numerator
in both of these regional data sets is usually limited to the neonatal
period, rather than the primary hospitalisation.
The Australian and New Zealand Neonatal Network collects data from
all the Australian and New Zealand Neonatal Intensive Care
Units.10 In this data set, the
denominator is limited to admissions to neonatal units, eliminating
livebirths who die outside the neonatal unit, and hence augmenting
the reported survival rates. Moreover, this data set is limited to
infants up to 31 weeks' gestation and excludes those with lethal
malformations. The numerator, however, includes deaths in the
neonatal period and those beyond 28 days of age that occur during the
primary hospitalisation.
Comparing data on cause of death is also difficult. Most government
data collection sources, such as those in Victoria7-9 and other
States, rely on confidential reports. They may not obtain enough data
to classify the cause of death, and rarely can they confirm the
gestational age. The final report includes an individual infant
under only one cause of death, even though there may be several equally
contributing causes of death, such as the common combination of HMD
and airleak, which caused 25% of deaths from non-lethal causes in our
study.
Our system of clinicopathological conferences for each death,
although not perfect, provided more detailed information about
causes of death.
As regional data sources do not provide complete data on deaths during
the primary hospitalisation at all gestational ages, we cannot
compare survival rates for preterm infants (32-36 weeks) with those
of term infants (37-42 weeks). However, we have calculated rates of
neonatal survival in Victoria for livebirths free of lethal
malformations with birthweight greater than 2499 g, assuming that
the results would be similar for infants of 37-42 weeks' gestation. In
our hospital, the neonatal survival rate for infants of 32-36 weeks'
gestational age (99.77%) approached the neonatal survival rate
expected of term infants in Victoria (99.95%).
The commonest causes of death were lethal anomalies, sepsis, and HMD.
Deaths from lethal anomalies are over-represented in our hospital
compared with Victoria as a whole as most have been diagnosed
antenatally, and, in many cases, the mother has been transferred
before birth from another hospital for management because of the
fetal anomaly.
The death rate from infection is higher in our hospital than for
Victoria, but this is probably because infants in our hospital live
longer, and hence acquire infections, rather than dying soon after
birth from other problems related to prematurity. E. coli and
Group B streptococci remain the chief cause of septic deaths,
particularly soon after birth, consistent with the observations of
Isaacs et al.14 Staphylococcus species
are an increasingly frequent cause of late infections,15 and of late
deaths in our study.
HMD remains a leading cause of death for very preterm infants despite
the fact that most mothers receive antenatal corticosteroid therapy
and all infants receive exogenous surfactant. Improvements in
exogenous surfactant offer hope of reducing mortality further, but
other antenatal interventions, such as thyrotropin stimulating
hormone,16 have been disappointing
in large randomised controlled trials. HMD did not cause any deaths in
our hospital in more mature preterm infants (28-36 weeks). Within the
infants who died from HMD, the observation that those who also had an
airleak were not as growth restricted as those who had no airleak might
reflect some structural change within the lung or with use of
pulmonary surfactant that is associated with growth restriction.
The problem of extreme intrauterine growth restriction is
highlighted by the deaths from pulmonary haemorrhage in three
infants, all with birthweight ratios below 0.55. The pulmonary
haemorrhage was not caused by failure to give intramuscular vitamin K
at birth.17 The mechanism for fatal
pulmonary haemorrhage is unclear, but may represent acute left heart
failure, contributed to by a patent ductus arteriosus. The
association of pulmonary haemorrhage and fetal growth restriction
is well known and may be related to histological changes in the ductus
arteriosus in growth-restricted fetuses.18 Pulmonary haemorrhage is
also seen more frequently with exogenous surfactant
therapy,19 which was given to two of
the three infants in our study who died of pulmonary haemorrhage.
Preventing prematurity, an obvious solution to the problem of higher
death rates in preterm infants, remains an elusive goal. In all
infants who died, few obvious preventable factors, apart from
avoiding preterm birth, were evident. The Annual Report from the
Victorian Consultative Council on Obstetric and Paediatric
Mortality and Morbidity consistently identifies more avoidable
factors in stillbirths than neonatal deaths.7-9
In summary, mortality rates fell sharply between 23 and 28 weeks'
gestational age, and few infants of more than 28 weeks' gestational
age without lethal anomalies died. Survival rates for preterm
infants of more than 31 weeks' gestational age approached the
survival rates expected of term infants. There were few obvious
avoidable factors in the deaths of any of the infants who died, either
in the very preterm infants of 23-27 weeks' gestational age, or the
more numerous preterm infants of 28-36 weeks' gestational age.
|
| |
References |
- The Victorian Infant Collaborative Study Group. Outcome at 2 years
of children 23-27 weeks' gestation born in Victoria in 1991-92. J
Paediatr Child Health 1997; 33: 161-165.
-
Crowley P. Corticosteroids before preterm delivery (Cochrane
Review). In: The Cochrane Library, Issue 2. Oxford: Update Software,
1998. [Updated quarterly.]
-
Soll RF. Natural surfactant extract vs synthetic surfactant in the
treatment of established respiratory distress syndrome (Cochrane
Review). In: The Cochrane Library, Issue 2. Oxford: Update Software,
1998. [Updated quarterly.]
-
Doyle LW, Davis P, Dharmalingam A, Bowman E. Assisted ventilation
and survival of extremely low birthweight infants. J Paediatr
Child Health 1996; 32:138-142.
-
Beeby PJ, Bhutap (sic) T, Taylor LK. New South Wales
population-based birthweight percentile charts. J Paediatr
Child Health 1996; 32: 512-518.
-
Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following
respirator therapy of hyaline- membrane disease: bronchopulmonary
dysplasia. N Engl J Med 1967; 276: 357-368.
-
The Consultative Council on Obstetric and Paediatric Mortality
and Morbidity. Annual report for the year 1994. Melbourne:
Department of Human Services, 1995.
-
The Consultative Council on Obstetric and Paediatric Mortality
and Morbidity. Annual Report for the Year 1995: Incorporating the
34th Survey of Perinatal Deaths in Victoria. Melbourne: Department
of Human Services, 1996.
<http://hna.ffh.vic.gov.au/phb/hce/peri/ob95/index.htm>
Accessed 11 May 1999.
-
The Consultative Council on Obstetric and Paediatric Mortality
and Morbidity. Annual report for the year 1996. Melbourne:
Department of Human Services, 1997.
<http://hna.ffh.vic.gov.au/phb/hce/peri/rep_96/contents.htm>
Accessed 11 May 1999.
-
Donoghue DA. Australian and New Zealand Neonatal Network, 1994.
Sydney: AIHW National Perinatal Statistics Unit, 1996. [Neonatal
Network Series no. 1.]
-
SPSS for Windows version 6.1 [computer program]. Chicago: SPSS
Inc, 1994.
-
Moses LE, Emerson JD, Hosseini H. Analyzing data from ordered
categories. N Engl J Med 1984; 311: 442-448.
-
Lancaster P, Huang J, Lin M. Australia's mothers and babies 1993.
Sydney: AIHW National Perinatal Statistics Unit, 1996. [Perinatal
Statistics Series No. 3.]
-
Isaacs D, Barfield C, Clothier T, et al. Early-onset group B
streptococcal infections in Aboriginal and non- Aboriginal
infants. Med J Aust 1995; 163: 302-306.
-
Isaacs D, Barfield C, Clothier T, et al. Late-onset infections of
infants in neonatal units. J Paediatr Child Health 1996;
32:158-161.
-
Actobat Study Group. Australian collaborative trial of
antenatal thyrotropin-releasing hormone (ACTOBAT) for prevention
of neonatal respiratory disease. Lancet 1995; 345: 877-882.
-
Loughnan PM, McDougall PN, Balvin H, et al. Late onset
haemorrhagic disease in premature infants who received intravenous
vitamin K1. J Paediatr Child Health 1996; 32: 268-269.
-
Ibara S, Tokunaga M, Ikenoue T, et al. Histologic observation of
the ductus arteriosus in premature infants with intrauterine growth
retardation. J Perinatol 1994; 14: 411-416.
-
Raju TN, Langenberg P. Pulmonary hemorrhage and exogenous
surfactant therapy: a metaanalysis. J Pediatr 1996; 123:
603-610.
(Received 29 Jun 1998, accepted 25 Mar 1999)
|
| | Authors' details |
Division of Paediatrics, The Royal Women's Hospital, Melbourne,
VIC.
Lex W Doyle, MD, MSc, FRACP, Paediatrician, and Associate
Professor, Department of Obstetrics and Gynaecology, University of
Melbourne;
Sheryle Rogerson, MB BS, Paediatric Fellow;
Shu-Ling Chuang, MB BCh, MRCP, Paediatric Fellow;
Matthew James, MB ChB, MRCP, Paediatric Fellow;
Ellen D
Bowman, MB BS, FRACP, Paediatrician;
Peter G Davis, MD,
BS, FRACP, Paediatrician.
Reprints will not be available from the authors.
Correspondence:
Associate Professor Lex Doyle, Department of Obstetrics and
Gynaecology, University of Melbourne, Parkville, VIC 3052.
Email: l.doyle@obgyn-rwh.unimelb.edu.au
©MJA 1999
| |
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia <http://www.mja.com.au>".
<URL: http://www.mja.com.au/>
© 1999 Medical Journal of Australia.
|
| | |
1: Causes of death defined
Lethal congenital anomalies: Any infants with major malformations that were considered untreatable or who died despite attempts at surgical correction, and infants with untreatable inborn errors of metabolism, chromosomal abnormalities, or overwhelming congenital infection acquired early in pregnancy.
Complications of prematurity:
Hyaline membrane disease (HMD), with or without an airleak: clinical signs of respiratory distress in the first days after birth, supported by radiological and/or autopsy evidence. Airleak comprised any air not confined to normal airspaces, such as pulmonary interstitial emphysema or pneumothorax.
Bronchopulmonary dysplasia (BPD): respiratory distress beyond four weeks of age requiring oxygen therapy, and radiographic changes typical of bronchopulmonary dysplasia,6 or a pathological diagnosis of bronchopulmonary dysplasia at autopsy.
Necrotising enterocolitis (NEC): confirmed by definite radiological, operative, or autopsy evidence.
Cerebroventricular haemorrhage (CVH): included any evidence of haemorrhage of any degree identified by ultrasound examination or autopsy. Cerebroventricular haemorrhage was considered a substantial contributor to death when knowledge of an intracerebral haemorrhage (grade 4) led to withdrawal of intensive care and the infant subsequently died.
Cystic periventricular leukomalacia (CPVL): included any cystic degeneration within the cerebral cortex. Cystic periventricular leukomalacia was considered the cause of death when it was severe enough to lead to the withdrawal of intensive care.
Ultrasound scanning was routine within the first three days after birth, at the end of the first week, then monthly until discharge as a minimum.
Pulmonary haemorrhage was diagnosed in infants with frothy, blood-stained tracheal fluid and clinical deterioration in respiratory function, or at autopsy.
Perinatal asphyxia: Infants who were liveborn but failed to respond adequately to resuscitation at birth.
Neonatal sepsis: Infants who died with clinical signs of sepsis supported by positive blood or cerebrospinal fluid (CSF) culture, but also included the occasional infant in whom sepsis was considered likely, but in whom blood or CSF cultures were sterile because of prior treatment with antibiotics, most commonly via the mother before birth. Pneumonia was diagnosed in infants with respiratory distress, signs of sepsis and a chest x-ray consistent with pneumonia, or at autopsy.
|
|
| | Back to text | | 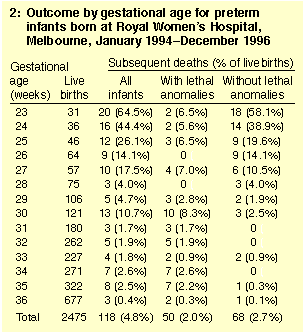 | | Back to text | | 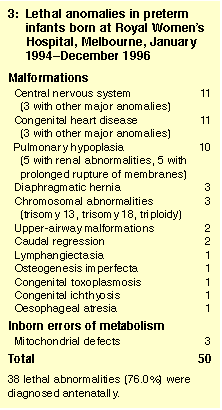 | | Back to text | | |
4: Causes of death in 68 preterm infants without lethal anomalies born at Royal Women's Hospital, Melbourne, January 1994-December 1996 | | | Median gestational | Median | | Major | Number | age in weeks | birthweight | | cause* | (%) | (range) | (range) |
| | Not offered intensive care | 7 (10.3%) | 23 (23-23) | 580 (545-725) | | Perinatal asphyxia | 5 (7.6%) | 26 (24-28.5) | 725 (630-1322) | Sepsis | 27 (39.7%) | 25 (23-26) | 751 (606-896) | | NEC | 5 (7.4%) | 24 (23-29.5) | 756 (610-916) | HMD | 23 (33.8%) | 24 (23-26) | 600 (522-750) | | HMD with airleak | 17 (25.0%) | 24 (23-25.5) | 670 (561-853) | | HMD without airleak | 6 (8.8%) | 25.5 (23-27) |
509 (371-582) | | BPD | 7 (10.3%) | 26 (24-28) | 711 (640-751) | | CPVL | 4 (5.9%) | 25 (24-27) | 765 (568-858) | | Pulmonary haemorrhage | 3 (4.4%) | 27 (27-30)¶ | 390 (315-756)¶ | | Other** | 5 (7.4%) | | | | Total | 68 | 25 (23-27) | 721 (590-856) | | | | | | | | | | | | Median | Median days of | | | Major | birthweight ratio | age at death | | | cause* | (range) | (range) | |
| | Not offered intensive care | 1.03 (0.94-1.23) | 1 (1-1) | | | Perinatal asphyxia | 0.96 (0.83-1.13) | 1 (1-1) | | Sepsis | 1.00 (0.90-1.09) | 8 (1-15) | | | NEC | 1.02 (0.68-1.16) | 14 (10-22.5) | | HMD | 0.92 (0.82-1.07) | 2 (1-4) | | | HMD with airleak | 0.99 (0.89-1.13) | 2 (1-3.5) | | | HMD without airleak | 0.66 (0.36-0.92) | 1.5 (1-5) | | | BPH | 0.80 (0.66-0.92) | 38 (19-141) | | | CPVL | 0.82 (0.76-1.13) | 49 (22-55) | | | Pulmonary haemorrhage | 0.37 (0.30-0.54)¶ | 5 (3-5)¶ | | | Other** | | Total | 0.95 (0.82-1.07) | | |
| * Some infants had more than one major cause of death.
 Range = interquartile range. Range = interquartile range. | BPD = bronchopulmonary dysplasia |  Includes five infants who died with NEC. Six also had Includes five infants who died with NEC. Six also had
HMD, two had both HMD and CVH, and two had CVH. | CPVL = cystic periventricular leukomalacia |  Three infants also had CVH and two had pulmonary Three infants also had CVH and two had pulmonary
haemorrhage. | CVH = cerebroventricular haemorrhage | | ¶ Range = complete range. | HMD = Hyaline membrane disease | ** Two with cardiomyopathy following twin-twin transfusion syndrome, one spontaneous gut perforation,
one neuroblastoma, one sudden infant death syndrome.
| NEC = necrotising enterocolitis | | - Infants not offered intensive care were significantly less mature than those who died after intensive care (median gestational age 23, interquartile range 23-23 v. median gestational age 25, interquartile range 24-27; z = 3.0, P < 0.01).
- Excluding those with lethal anomalies and those not offered intensive care, children dying with HMD were less mature than those dying without HMD (median gestational age 24, interquartile range 23-26 v. median gestational age 26, interquartile range 24-28;
z = 2.9, P < 0.01) and lighter at birth (median birthweight 600 g, interquartile range 522-750 g v. median birthweight 758 g, interquartile range 683-888 g; z = 3.0, P < 0.01), but had similar birthweight ratios (median 0.92, interquartile range 0.82-1.07 v. median 0.96, interquartile range 0.81-1.05, not significant).
- Within the group of infants who died of
HMD, those who also had airleak were not significantly different in maturity, but were significantly heavier (z = 2.7, P < 0.01) and had higher birthweight ratios (z = 2.5, P < 0.02).
- The three infants who died of pulmonary haemorrhage were particularly growth restricted, with birthweight ratios of 0.30, 0.37, and 0.54, respectively; their median birthweight ratio of 0.37 was substantially below that of the infants who died of other causes (median birthweight ratio 0.96, interquartile range 0.85-1.07; z = 2.8, P < 0.01).
- Most infants who died did so soon after birth, except those who died of sepsis, BPD,
or CPVL.
- Infants who died of sepsis were generally
quite immature and small at birth, but were not particularly growth restricted.
- In infants without NEC who died of sepsis, the infectious organisms were Escherichia coli (8), Group B streptococci (3), Staphylococcus aureus(5), S epidermidis (1), Haemophilus influenzae (1), Klebsiella and Pseudomonas spp. combined (1), Streptococcus viridans (1), Candida albicans (1) and herpes simplex (1).
E. coli and Group B streptococci were the predominant organisms causing early infections, and Staphylococcus species,
later infections.
|
|
| | Back to text | | 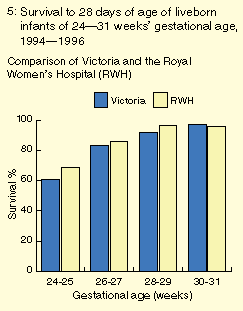 | | Back to text | | 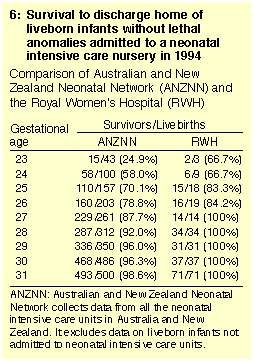 | | Back to text |
|








