On 24 and 31 October 2013, the Australian Broadcasting Corporation (ABC) aired a two-part special edition of the science journalism series, Catalyst, titled Heart of the matter, that was critical of HMG-CoA reductase inhibitors (“statins”). The program questioned the link between high cholesterol levels and cardiovascular disease, and suggested that the benefits of statins had been overstated and the harms downplayed.1 Nearly 1.5 million Australians are estimated to have viewed each part of the program.2
Statins are recommended nationally and internationally both for primary prevention of cardiovascular events in people at increased risk of cardiovascular disease, and for secondary prevention in those with established cardiovascular disease.3,4 They are the most commonly prescribed medicines in Australia,5 used by over 30% of the population aged 50 years and older.6
Considerable media debate and backlash from the medical community followed the Catalyst program, including criticism for misleading patients.2,7 A National Heart Foundation survey of 1094 patients treated with lipid-modifying medications found that 11% of patients who watched Catalyst reported ceasing to take their cholesterol medicines, an additional 12% stopped taking them but restarted, and 12% reported starting to use “natural remedies”.8 Moreover, a survey by the Australian Capital Territory Government of general practitioners and pharmacists found that 58% reported that some of their patients had stopped taking their statins after the Catalyst program.9 Our purpose in this study was to quantify any changes in the dispensing of statins after the airing of the Catalyst program in October 2013.
Methods
We used dispensing records from the Pharmaceutical Benefits Scheme (PBS) — under which all citizens and permanent residents of Australia are entitled to subsidised access to prescribed medicines — from 1 July 2009 to 30 June 2014 for a 10% random sample of people who were dispensed a PBS-listed medicine. The 10% PBS sample is a standard dataset provided by the Australian Government Department of Human Services for analytical use, and is selected based on the last digit of each individual's randomly assigned unique identifier. This dataset captures all dispensed PBS-listed medicines attracting a government subsidy, which occurs when the price of the medicine is above the PBS copayment threshold. To protect the privacy of people in this dataset, all dates of dispensing are offset randomly by + 14 or − 14 days; the direction of the offset is the same for all records for each individual.
We restricted our analyses to people for whom we had a complete PBS dispensing history for the entire study period. As many commonly dispensed statins fall below the general copayment threshold ($36.90 at 1 January 2014), but above the concessional copayment threshold ($6.00), we included only long-term concessional beneficiaries (ie, individuals dispensed only medicines attracting a concessional copayment during the 5-year study period). Long-term concessional beneficiaries represent about 51% of all people who are dispensed a statin, and consist of older people, those on a low income and the sick and disabled.
Medicines of interest
We included all doses (including combination products) of PBS-listed statins (atorvastatin, fluvastatin, pravastatin, rosuvastatin and simvastatin) and proton pump inhibitors (PPIs; esomeprazole, lansoprazole, omeprazole, pantoprazole and rabeprazole). PPI dispensing was chosen as the comparator as these medicines are commonly dispensed and are used by a similar population as use statins. In addition, we expected that PPI dispensing would be unlikely to be affected by the Catalyst program.
Measures
We defined discontinuation as the absence of any dispensing for a period of at least three times the number of pills last dispensed (assuming one pill per day) plus a 5-day grace period. Nearly all statin dispensing records (99.5%) were for a 30-day supply; therefore, in most cases, a period of 105 days or more without a statin dispensing record was considered a discontinuation. The date of discontinuation was the date that patients would have been expected to refill their prescriptions plus a 5-day grace period (ie, date of last dispensed statin + 35 days).
We classified individuals into four mutually exclusive risk categories. These were based on medicines dispensed for the treatment of cardiovascular disease (World Health Organization Anatomical Therapeutic Chemical [WHO ATC] codes C [excluding statins and topical agents for treating venous disorders] and B01AC), and diabetes (WHO ATC code A10). These risk categories were: (i) dispensed no other cardiac medicines and no diabetes medicines; (ii) dispensed 1–2 other cardiac medicines and no diabetes medicines; (iii) dispensed ≥ 3 other cardiac medicines and no diabetes medicines; or (iv) dispensed at least one diabetes medicine.
Statistical analysis
We used an interrupted time-series analysis to assess the impact of the Catalyst program on dispensing and discontinuation of statins. The date of the first part of the Catalyst program (24 October 2013) was the change point. For each week, we summed the number of dispensing records and the number of individuals who discontinued for statins and for PPIs, overall and stratified by risk category (statins only). We defined a week as starting on Thursday, the day that Catalyst aired. Data were log-transformed to estimate the percentage change.
PBS dispensing data are highly seasonal;10 once an individual or family's out-of-pocket PBS expenses exceed the PBS Safety Net threshold for a calendar year, all PBS medicines have a reduced copayment until 31 December of that year. To account for this seasonal variability, as well as long-term trends and autocorrelation, we modelled the time series using an autoregressive integrated moving average (ARIMA) approach, using the Box–Jenkins method11 (Appendix). We created individual ARIMA models for overall dispensing (statins and PPIs), for discontinuation (statins and PPIs), and for dispensing and discontinuation within each risk category (statins only).
We estimated the average number of dispensings of statins per week in Australia (including statins falling below the copayment) in the 3 months before the Catalyst program aired using publicly available aggregated dispensing data.12 These data were used to estimate the impact of the Catalyst program on all statin users, not just people included in the 10% PBS sample.
All analyses were performed in SAS, version 9.3 (SAS Institute Inc), and Stata, version 12 (Statacorp).
The study was approved by the New South Wales Population and Health Services Ethics Committee (2013/11/494) and the Department of Human Services External Request Evaluation Committee.
Results
In our sample, 191 833 people were dispensed a statin from 1 July 2009 to 30 June 2014, with a mean of 26 946 statin dispensings weekly (range, 23 505 to 30 465). The average age of statin users in our study sample in 2013 was 72 years (SD, 12 years), and 55% were women. Thirteen per cent of our study population were dispensed no other cardiac or diabetes medicines, 25% were dispensed 1–2 cardiac medicines and no diabetes medicines, 36% were dispensed ≥ 3 cardiac medicines and no diabetes medicines, and 27% were dispensed diabetes medicines (93% of whom were also dispensed at least one cardiac medicine).
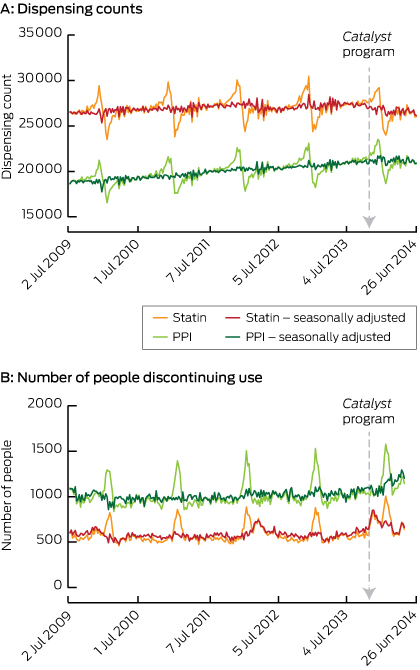
The overall trend in dispensing was relatively stable, with the highest dispensing counts in November and December, and lowest in January and February owing to individuals reaching their safety net threshold and preferentially refilling their prescriptions more frequently at the end of the calendar year. Raw dispensing counts and counts adjusted for seasonal variation are presented in Box 1 (A). Rates of discontinuation follow a similar pattern, with an increase at the beginning of every year Box 1 (B).
Dispensing
The week the Catalyst program was aired, we found a significant sustained change of 2.60% fewer statin dispensings per week (Box 2). Given that there are an average of 538 640 statin dispensings per week in Australia,12 this corresponds to an estimated decrease of 14 005 dispensings per week in the Australian population. Assuming that most users are dispensed statins once a month, the equivalent of 60 897 Australians would be affected.
We found a significant reduction in the rate of statin dispensings in all risk categories (Box 2), with 6.0% fewer statin dispensings per week to people with no evidence of taking other cardiac or diabetes medicines and 1.9% fewer dispensings to those with evidence of taking diabetes medicines.
We also identified a decrease in statin dispensing of 1.96% (95% CI, 1.12%–2.79%; P < 0.001) starting the week of 1 March 2012, which coincided with the publication of a news story about the risk of diabetes and dementia associated with statin use;13 the level of statin dispensing returned to expected levels in March 2013.
We found no significant change in the number of PPIs dispensings in the period following the Catalyst program (0.08%; 95% CI, − 1.53% to 1.71%; P = 0.92).
Discontinuation
The number of people discontinuing their use of statins increased by 28.8% (P < 0.001) in the week that the Catalyst program aired, and this effect decayed by 9% (P < 0.001) per week, returning to average levels after 18 weeks (Box 3). On average, 1.8% of statin users discontinued using statins each month before the Catalyst program aired; thus, following the Catalyst program, an estimated 28 784 additional Australians discontinued their use of statins. A significant increase in discontinuation was observed regardless of the use of other cardiovascular and diabetes medications (Box 3).
In addition, we observed an increase in discontinuation after the 2012 news story,13 peaking at 29.0% (95% CI, 19.1%–39.6%; P < 0.001) and decaying by 8% each week thereafter, lasting 21 weeks.
There was no apparent change in PPI discontinuation following the airing of the Catalyst program.
Discussion
We found significant and sustained changes in statin dispensing following the airing of the Catalyst program — 2.6% fewer statins were dispensed every week (a total of 504 180 fewer dispensings of statins), which equates to 60 897 Australians having been affected up to 30 June 2014, as a result of increased discontinuation, decreased initiation and/or poor adherence. This includes an estimated 28 784 additional people who discontinued their statins.
On average, among all statin users, the number-needed-to-treat over 5 years to prevent one major vascular event such as a myocardial infarction or stroke ranges from 21 (for those with pre-existing coronary heart disease) to 40 (for those without).14 It is unclear how long the change in statin use that we observed is likely to last. If the 60 897 individuals we estimated to have been affected continue to be non-adherent, this could result in between 1522 and 2900 preventable, and potentially fatal, major vascular events. While statins have been shown to reduce cardiovascular events regardless of an individual's absolute cardiovascular risk,15 national guidelines recommend their use in those who have had a previous cardiovascular event and in those at moderate or high absolute cardiovascular risk.16 There is evidence that, in Australia, statins are both underused in those at high risk and overused in those at low risk.17 Some of the observed reduction in use may result from patients at low absolute risk of cardiovascular disease ceasing therapy.
However, individuals who did and did not concomitantly take cardiovascular and diabetes medications had reduced their use of statins after the Catalyst program aired. This includes individuals likely to be at high cardiovascular risk, such as those with diabetes; the proven and substantial efficacy of statins in this group means they can least afford to discontinue therapy.18,19
Many elements of the Catalyst program's contents were inconsistent with the recommendations of key medical advice about statins and cardiovascular disease3 and the ABC has since withdrawn the program, primarily on the grounds that it breached their impartiality standards.20 The program was watched by a substantial proportion of the Australian public and is likely to have influenced their beliefs about the risks and benefits of statins and the relationship between high cholesterol and cardiovascular disease. Belief in the effectiveness of medication and the need for treatment are important predictors of adherence to lipid-lowering medications.21 The National Heart Foundation's survey of users of lipid-modifying agents found that, compared with those who were unaware of the program, those who watched it were more likely to express concerns about taking their cholesterol medicines, and a desire to stop.8
Our study is not the first to show the impact of adverse media reports on prescribed medicine use in Australia. A 2007 television news program about the association between osteonecrosis of the jaw and bisphosphonate use was associated with 29 633 fewer prescriptions.22 Further, our incidental finding of a reduction in statin dispensing in 2012 that coincided with a news story about the risks of diabetes and dementia demonstrates the long-lasting impact of such publicity; statin dispensing only returned to average levels after a year.
The strength of our study lies in the use of a representative sample of all Australians ever dispensed a PBS-subsidised medicine, and the use of a long-term concessional beneficiary population, ensuring complete capture of statin dispensing. Analyses of time-series data can be problematic because of high levels of autocorrelation, underlying trends, and seasonality. While PBS dispensing data are particularly seasonal, the ARIMA approach is well established and ideally suited for dealing with these problems.11,23 Further, although the lack of change in PPI dispensing supports the idea that the changes in statin dispensing were in response to the Catalyst program, we cannot rule out other factors affecting dispensing behaviour during this study period. We also saw no change in non-statin lipid-lowering medicines (data not shown). We also did not know the exact dates of dispensing, as all dates were offset by 2 weeks, but this would be expected to bias our findings towards the null. Lastly, while we categorised individuals based on other cardiovascular and diabetes medicines they were dispensed, it is not possible to know their true level of cardiovascular risk without additional information, such as blood pressure and smoking status, which was not available in our study.
We estimated the change in the use of statins after the airing of the Catalyst program in all Australians who were prescribed statins based on findings in our study sample. However, as the Australian non-concession population is younger, has higher socioeconomic status, and is in better health than the long-term concession population, they probably have a lower risk of cardiovascular events and a higher risk of not adhering to their statin regimen. Consequently, we may have underestimated the population-level impact on dispensing.
As of mid 2014, there is no indication that the change in dispensing after the Catalyst program has abated. Even though the observed effect was relatively small, the prevalence of statin use in Australia and the established efficacy of these drugs14,22 means that a large number of people are affected, and may suffer unnecessary consequences. The changes in statin use occurred despite warnings in the Catalyst program that its content should not be taken as medical advice, and public criticism of the program. The subsequent retraction of the program may counteract some of the apparent negative impact, but this remains to be seen.
2 Autoregressive integrated moving average (ARIMA) modelling results* of the impact of the Catalyst program on statin dispensing
Population | Mean weekly dispensings 12 weeks before Catalyst | Weekly change in number of dispensings | |||||||||||||
% (95% CI) | P | ||||||||||||||
Overall | 27 536 | − 2.60 (− 3.77 to − 1.40) | < 0.001 | ||||||||||||
No cardiac or diabetes medicines | 2 549 | − 6.03 (− 8.28 to − 3.73) | < 0.001 | ||||||||||||
1–2 cardiac medicines and no diabetes medicines | 6 602 | − 2.77 (− 4.54 to − 1.06) | 0.002 | ||||||||||||
≥ 3 cardiac medicines and no diabetes medicines | 10 252 | − 2.40 (− 3.34 to − 1.46) | < 0.001 | ||||||||||||
Diabetes medicines | 8 133 | − 1.94 (− 3.45 to − 0.42) | 0.01 | ||||||||||||
* ARIMA specification, (3,1,1)(3,1,1)52. | |||||||||||||||
3 Autoregressive integrated moving average (ARIMA) modelling results of the impact of the Catalyst program on the number of people who discontinued their use of statins
Population | Mean weekly number of discontinuers 12 weeks before Catalyst | Peak change in discontinuation | Weekly | Duration of effect* | |||||||||||
Time to peak | % (95% CI) | P | |||||||||||||
Overall† | 576 | 0 weeks | 28.8% (15.4% to 43.7%) | < 0.001 | 9% | 18 weeks | |||||||||
No cardiac or diabetes medicines† | 87 | 0 weeks | 72.2% (27.3% to 133.0%) | < 0.001 | 16% | 16 weeks | |||||||||
1–2 cardiac medicines and no diabetes medicines† | 143 | 0 weeks | 58.4% (28.6% to 95.1%) | < 0.001 | 12% | 20 weeks | |||||||||
≥ 3 cardiac medicines and no diabetes medicines‡ | 186 | 1 week | 30.5% (11.1% to 53.3%) | 0.001 | 13% | 14 weeks | |||||||||
Diabetes medicines† | 161 | 2 weeks | 38.6% (13.3% to 69.6%) | 0.002 | 40% | 5 weeks | |||||||||
* The impact was considered to have ended when it decayed to ≤ 5%. † ARIMA specification, (0,1,1)(0,1,1)52. ‡ ARIMA specification, (0,1,2)(0,1,2)52. | |||||||||||||||
Received 29 January 2015, accepted 23 April 2015
- Andrea L Schaffer1
- Nicholas A Buckley1
- Timothy A Dobbins1
- Emily Banks2
- Sallie-Anne Pearson1
- 1 University of Sydney, Sydney, NSW.
- 2 Australian National University, Canberra, ACT.
This research is supported, in part, by a National Health and Medical Research Council (NHMRC) Health Services Research Capacity Building Grant (ID: 571926) and funding from the NHMRC Centre of Research Excellence in Medicines and Ageing (ID: 1060407). Sallie-Anne Pearson is supported by a Cancer Institute New South Wales Career Development Fellowship (ID: 12/CDF/2-25) and is an Australian Health Policy Research Fellow. Andrea Schaffer and Emily Banks are supported by the NHMRC (IDs: 1074924 and 1042717, respectively). We thank the Department of Human Services for providing the data for this research.
Emily Banks made statements in the media about the potential public health impact at the time of the program's airing. No other authors have relevant disclosures.
- 1. Australian Broadcasting Corporation. Catalyst. Heart of the matter. Sydney: ABC, 2014. http://www.abc.net.au/catalyst/heartofthematter (accessed Apr 2015).
- 2. Australian Broadcasting Corporation. Media Watch. Catalyst challenges the mainstream. Sydney: ABC, 2013. http://www.abc.net.au/mediawatch/transcripts/s3888657.htm (accessed Jan 2015).
- 3. National Heart Foundation of Australia. The National Heart Foundation of Australia's summary of the recommendations for cholesterol management. Canberra: NHF, 2014. http://www.heartfoundation.org.au/SiteCollectionDocuments/Heart%20Foundation%20summary%20of%20recommendations%20for%20cholesterol%20management%20-%20Catalyst%20-%20FINAL.pdf (accessed Mar 2014).
- 4. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: 2889-2934.
- 5. Australian Government Department of Health. Australian Statistics on Medicines 2011. Canberra: Department of Health, 2013. http://www.pbs.gov.au/statistics/asm/2011/australian-statistics-on-medicines-2011.pdf (accessed Jul 2014).
- 6. Morgan TK, Williamson M, Pirotta M, et al. A national census of medicines use: a 24-hour snapshot of Australians aged 50 years and older. Med J Aust 2012; 196: 50-53. https://www.mja.com.au/journal/2012/196/1/national-census-medicines-use-24-hour-snapshot-australians-aged-50-years-and. (accessed May 2014). <MJA full text>
- 7. National Heart Foundation of Australia. Cholesterol still important risk factor for heart disease. Sydney: NHF, 2013. http://www.abc.net.au/mediawatch/transcripts/1341_heartfoundation1.pdf (accessed Mar 2014)
- 8. National Heart Foundation of Australia. The impact of ‘ABC Catalyst' on lipid modifying medication use. Sydney: NHF, 2013. http://www.heartfoundation.org.au/news-media/Media-Releases-2013/Documents/The%20Impact%20of%20ABC%20Catalyst%20on%20Lipid%20Modifying%20Medication%20Use%20Survey%202013.pdf (accessed Dec 2014)
- 9. Australian Capital Territory Medicare Local. Statin use survey results. Canberra: ACTML, 2014. http://www.actml.com.au/Uploads/Documents/Statin%20use%20survey%20results_20140401115619.pdf (accessed Jan 2015).
- 10. Donnelly N, McManus P, Dudley J, Hall W. Impact of increasing the re-supply interval on the seasonality of subsidised prescription use in Australia. Aust N Z J Public Health 2000; 24: 603-606.
- 11. Box GEP, Jenkins GM, Reinsel GC. Time series analysis: forecasting and control. 4th ed. Hoboken, NJ: John Wiley, 2008.
- 12. Australian Government Department of Health. PBS and RPBS Section 85 Date of Processing and Date of Supply Data. Canberra: Department of Health, 2014. http://www.pbs.gov.au/info/statistics/dos-and-dop/dos-and-dop (accessed Jan 2015).
- 13. Corderoy A. “Miracle” drugs put thousands at risk. Sydney Morning Herald 2012; 1 Mar. http://www.smh.com.au/national/health/miracle-drugs-put-thousands-at-risk-20120229-1u3ia.html (accessed Nov 2014).
- 14. Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366: 1267-1278.
- 15. Cholesterol Treatment Trialists' (CTT) Collaborators; Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380: 581-590.
- 16. National Vascular Disease Prevention Alliance. Guidelines for the management of absolute cardiovascular disease risk. Sydney: National Stroke Foundation, 2012. http://strokefoundation.com.au/site/media/AbsoluteCVD_GL_webready.pdf (accessed Mar 2015).
- 17. Heeley EL, Peiris DP, Patel AA, et al. Cardiovascular risk perception and evidence–practice gaps in Australian general practice (the AusHEART study). Med J Aust 2010; 192: 254-259. <MJA full text>
- 18. Wei L, Ebrahim S, Bartlett C, et al. Statin use in the secondary prevention of coronary heart disease in primary care: cohort study and comparison of inclusion and outcome with patients in randomised trials. BMJ 2005; 330: 821.
- 19. Cholesterol Treatment Trialists' (CTT) Collaborators; Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371: 117-125.
- 20. Australian Broadcasting Corporation. Catalyst ‘Heart of the Matter' Investigation Report. Canberra: ABC, 2014. http://about.abc.net.au/wp-content/uploads/2014/05/Catalyst-Heart-of-the-Matter-ACA-Investigation-Report.pdf (accessed Dec 14).
- 21. Casula M, Tragni E, Catapano AL. Adherence to lipid-lowering treatment: the patient perspective. Patient Prefer Adherence 2012; 6: 805-814.
- 22. Sambrook PN, Chen JS, Simpson JM, March LM. Impact of adverse news media on prescriptions for osteoporosis: effect on fractures and mortality. Med J Aust 2010; 193: 154-156. <MJA full text>
- 23. Lagarde M. How to do (or not to do).. assessing the impact of a policy change with routine longitudinal data. Health Policy Plan 2012; 27: 76-83.





Abstract
Objectives: To examine the impact of a two-part special edition of the Australian Broadcasting Corporation's science journalism program Catalyst (titled Heart of the matter), aired in October 2013, that was critical of HMG-CoA reductase inhibitors (“statins”).
Design, setting and participants: Population-based interrupted time-series analysis of a 10% sample of Australian long-term concessional beneficiaries who were dispensed statins under the Pharmaceutical Benefits Scheme (about 51% of all people who were dispensed a statin between 1 July 2009 and 30 June 2014); dispensing of proton pump inhibitors (PPIs) was used as a comparator.
Main outcome measures: Change in weekly dispensings and discontinuation of use of statins and PPIs, adjusting for seasonal and long-term trends, overall and (for statins only) stratified by the use of cardiovascular and diabetes medicines.
Results: In our sample, 191 833 people were dispensed an average of 26 946 statins weekly. Following the Catalyst program, there was a 2.60% (95% CI, 1.40%–3.77%; P < 0.001) reduction in statin dispensing, equivalent to 14 005 fewer dispensings Australia-wide every week. Dispensing decreased by 6.03% (95% CI, 3.73%–8.28%; P < 0.001) for people not dispensed other cardiovascular and diabetes medicines and 1.94% (0.42%–3.45%; P = 0.01) for those dispensed diabetes medicines. In the week the Catalyst program aired, there was a 28.8% (95% CI, 15.4%–43.7%; P < 0.001) increase in discontinuation of statin use, which decayed by 9% per week. An estimated 28 784 additional Australians ceased statin treatment. Discontinuation occurred regardless of the use of other cardiovascular and diabetes medicines. There were no significant changes in PPI use after the Catalyst program.
Conclusions: Following airing of the Catalyst program, there was a temporary increase in discontinuation and a sustained decrease in overall statin dispensing. Up until 30 June 2014, there were 504 180 fewer dispensings of statins, and we estimate this to have affected 60 897 people.