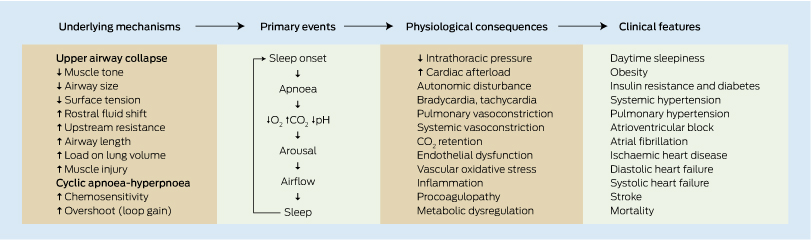
Patients with obstructive sleep apnoea (OSA) have a high prevalence of insulin resistance (IR), type 2 diabetes mellitus and cardiovascular disease (CVD), indicating a strong association among the conditions. Intermittent hypoxia with fragmentation of normal sleep contributes to significant autonomic dysfunction plus proinflammatory and procoagulopathy states,1 leading to IR and CVD (Box 1). Although obesity is a common risk factor for OSA, IR and particularly CVD, current evidence suggests that OSA itself is an independent risk factor for both IR and CVD. Clinical data suggest that this effect is most likely mediated via intermittent oxygen desaturation. However, teasing out the precise role that OSA plays in IR and CVD, independent of obesity, is difficult given the confounding effects of inactivity, sleep deprivation, diet and OSA variability in terms of age of onset, duration and severity. With this in mind, this paper attempts to review the epidemiological and interventional evidence connecting OSA with IR and CVD (Box 2).
Obesity is the major risk factor for OSA, particularly central adiposity with visceral fat. Large epidemiological studies have reported a dose–response association between OSA prevalence and increased body mass index (BMI) plus neck and waist circumferences. One large prospective epidemiological study reported that a 10% weight gain led to a sixfold increase in the odds of developing moderate to severe OSA, independent of confounding factors.22
Conversely, weight loss improved OSA, but to a lesser extent than weight gain worsened it (10% weight loss predicted a 26% decrease in the apnoea–hypopnoea index [AHI]). The latter observation underscores the potential for weight loss as a treatment for OSA. Observational data suggest an improvement in OSA with weight loss, although results from randomised controlled trials (RCTs) have been available only more recently. Trial data indicate that patients with mild OSA substantially improve their OSA with weight loss, although only 22% achieved a “cure” (AHI < 5/h).23 However, among obese patients with severe OSA, results from weight loss studies are more unpredictable. Data from lifestyle interventions show an improvement in OSA, with weight loss of at least 10 kg, but only a minority of patients achieved an AHI < 5/h.24,25
A recent Australian RCT assessing the effect of laparoscopic gastric banding surgery in morbidly obese patients with moderate to severe OSA showed that although surgical patients lost more weight, there was no significantly greater reduction in AHI in the surgical group compared with the control group who undertook lifestyle measures.26 In both groups, there were significant improvements in symptoms of sleepiness and mood, despite only about a 50% reduction in the group AHI, suggesting that weight loss per se, rather than OSA reversal, contributed to improved quality of life. Metabolic parameters also improved with weight loss and were greatest in the surgical group, who lost more weight. This trial underlines two important points. First, obese patients with mild OSA may be “cured” by weight loss, but those with moderate to severe OSA are rarely “cured” by either surgical or medical weight loss strategies. Second, many significant health benefits (relating to quality of life, depression and diabetes control) can be achieved by weight loss in obese OSA patients, even if OSA persists.
OSA and systemic hypertension commonly coexist — the prevalence of OSA in populations with systemic hypertension has been reported to vary from 30% to 83%.27 Several large epidemiological cross-sectional studies of community dwellers indicate that the presence of untreated OSA is associated with a greater prevalence of hypertension when controlled for known confounding factors,2 although the association is weaker in prospective incidence studies.3 Although some prospective incidence studies of middle-aged adults have found untreated OSA to be associated with a two- to threefold risk of developing hypertension over a 4–8-year period,4 not all studies have found a positive association between OSA and hypertension.3 In addition, the relationship between OSA and hypertension has not been confirmed in patients aged > 65 years,5 probably because of additional accumulating risk factors.
Treatment of OSA with continuous positive airway pressure (CPAP) has been shown to lead to reductions in mean systemic blood pressure measured over 24 hours, although these falls are small (about 2–3 mmHg), with the greatest benefit seen in patients with more severe OSA.6 There is also evidence that treatment with mandibular advancement splints leads to an improvement in hypertension,7 suggesting that the benefit of OSA treatment with respect to blood pressure is independent of the treatment modality.
Despite this, pharmacological antihypertensive therapy (valsartan) is more effective than CPAP (9 mmHg v 2 mmHg fall in mean 24-hour blood pressure) over 8 weeks, according to one RCT of 23 patients with hypertension and OSA.28
Four important messages need consideration regarding OSA and hypertension. First, clinicians should assess for OSA symptoms and consider a sleep study in patients with resistant hypertension.29 Second, OSA treatment in hypertensive OSA patients may improve blood pressure control but without large reductions, while snoring and quality of life should improve. Third, CPAP is not a substitute for pharmacological treatments. Fourth, obesity is a unifying factor and, accordingly, assistance with weight loss should be the primary objective for clinicians.
Metabolic disorders of glucose control and OSA share the same major risk factor of central obesity with excess visceral fat and, unsurprisingly, the disorders commonly coexist. Mechanistically, OSA may aggravate IR and type 2 diabetes via intermittent hypoxia, fragmented sleep and elevated sympathetic activity. Diabetes may contribute to OSA via neuropathy and weight gain related to insulin use. The prevalence of OSA in patients with type 2 diabetes has been reported to vary between 23%8 and 86%,9 with differences in study populations and OSA definitions explaining the marked variation in results.10 Most cross-sectional studies have demonstrated that OSA is independently associated with IR and type 2 diabetes in adult sleep clinic populations and in unselected communities, independent of age and BMI, but prospective incidence studies have been less convincing.
The effect of OSA treatment with CPAP on insulin sensitivity and glucose control (ie, HbA1c levels) is unclear. A recent meta-analysis11 of five RCTs (four with crossover design) suggested that reversal of OSA with CPAP for 1–12 weeks has a beneficial effect on IR, as measured by homoeostatic model assessment in OSA patients without diabetes, although the effect size was small (− 0.44) in contrast to pharmacological effects (− 0.9). The only RCT of CPAP treatment of OSA among adults with type 2 diabetes indicated that CPAP did not improve homoeostatic model assessment scores, HbA1c levels or BMI in 42 patients with moderate to severe OSA and type 2 diabetes, although patients were symptomatically and objectively less sleepy.30 A larger and longer international trial is nearing completion (NCT00509223). Some authors suggest the variable effects of CPAP on glucose control may relate to duration of CPAP treatment (potentially > 3 months) and that the effects may be greatest in patients who are less obese.31
Patients with untreated OSA have an elevated risk of developing stroke, and the data are more consistently positive than for cardiac disease,18 including in the elderly.32 Mechanisms include large swings in systemic blood pressure, local vibrational damage to the carotid artery bifurcation, increased coagulopathy, surreptitious development of atrial fibrillation during sleep with thrombus formation and paradoxical emboli through asymptomatic patent foramen ovale opening during transient sleep-related hypoxaemia with pulmonary hypertension.33
Prospective observational studies show increasing risk for ischaemic stroke with increasing OSA severity.19 A large epidemiological study in the United States found that the risk for stroke in men increased almost three times once the AHI was > 19/h, but that the risk in women was much smaller and did not become significant until AHI was > 25/h.19
CPAP treatment may reduce stroke risk; however, large RCTs are lacking. Observational studies have shown that treatment of OSA reduces stroke risk. The only RCT assessing the effect of CPAP on risk of mortality and subsequent stroke did not show a benefit, but had only small numbers and was not adequately powered to address the issue.34
The prevalence of OSA is high (estimated to be 30%–58%) in patients with ischaemic heart disease (IHD).12 In the general community, cross-sectional epidemiological evidence supports a link between OSA and IHD. OSA is associated with a greater risk for acute myocardial infarction than are smoking or hypertension.13 Further, the presence of OSA in patients with established IHD is associated with greater 7-year mortality compared with patients without OSA.12 Whether underlying OSA contributes to the well described circadian distribution of myocardial infarction (peak incidence around 8 am) remains to be determined. RCTs of OSA treatment on the development or outcomes of IHD are presently lacking.
Benign cardiac arrhythmias are commonly present in OSA. Examples include cyclic tachycardia–bradycardia, atrial and ventricular ectopics, bigeminy, heart block and atrial fibrillation.35 In a large study, subjects with severe OSA (AHI > 30/h) were found to be more likely to have atrial fibrillation (fourfold risk), non-sustained ventricular tachycardia (4.4-fold risk) and quadrigeminy (twofold risk) compared with subjects without OSA.14 The clinical significance of this is unknown. Similar data were provided for Australians with moderate OSA (AHI > 15/h), with an odds ratio of 3 for having atrial fibrillation.35
Some data suggest that all arrhythmias improve with CPAP treatment,36 whereas other data are not as supportive.37 One study suggested the 12-month recurrence of atrial fibrillation after cardioversion was significantly lower if coexistent OSA was treated with CPAP compared with untreated OSA.38
Although the risk of fatal arrhythmias from OSA is unknown, an increased risk is suggested from data showing that subjects with OSA who die of sudden cardiac death are more likely to do so at night compared with those without OSA.39
Both diastolic and systolic heart failure (HF) are common in OSA populations. In addition to the proposed effect of OSA on CVD, large swings in negative intrathoracic and positive intravascular pressures that result from OSA are thought to contribute to the development of cardiomyopathy as well as hypertension, hypoxia, hypoxic pulmonary hypertension and oxidative stress.1 Epidemiological data indicate a threefold greater prevalence of diastolic and systolic HF in community dwellers with severe OSA (AHI > 30/h) compared with those without OSA.15 Further, the risk of developing incident HF due to untreated OSA is estimated to be 1.6 times greater, based on 4422 community dwellers (controlled for age, sex, race, diabetes and hypertension) followed for a mean of 8.7 years.16
OSA and central sleep apnoea (defined by about 30 seconds of hyperventilation followed by about 30 seconds of apnoea with no respiratory effort and usually absence of snoring) are also commonly seen within HF populations. A study demonstrated that 55%–85% of HF patients have sleep apnoea (either obstructive or central) when patients were tested several times over a 12-month period.40 In general, central sleep apnoea is seen in the more advanced severe spectrum of HF and can be explained by additional pathophysiology to that seen in pure OSA. The high prevalence of each type of apnoea does not appear to have been affected by the introduction of β-blockers or spironolactone.41
Evidence suggests that coexistent OSA worsens HF and is improved by CPAP therapy. An RCT found that treatment of patients with OSA (AHI > 20/h) and systolic HF with fixed pressure CPAP over 3 months was associated with improvements in systolic function, quality of life, exercise capacity and autonomic control.17 Nevertheless, the data are not universally positive. The study was not large enough to assess mortality; however, an observational study suggests an improvement in survival with long-term CPAP treatment, compared with untreated OSA.42
Several large, longitudinal epidemiological studies have consistently indicated that in middle-aged populations, severe untreated OSA (AHI > 30/h) is associated with greater mortality compared with treated OSA, mild to moderate OSA or no OSA.20 These data suggest that severe OSA confers a mortality risk, which is prevented by CPAP treatment. Nevertheless, these studies were not RCTs and, given that unrecognised bias may confound the results, whether OSA is a reversible risk factor for mortality remains inconclusive.
The mortality effects of untreated OSA are less certain in the elderly. In a large cohort of 14 589 Israeli patients, severe OSA led to increased mortality only for those aged < 50 years.21 Similarly, a large US study also failed to show increased mortality in patients aged > 70 years.20 However, a recent Spanish observational trial reported that elderly patients (> 65 years of age) with severe untreated OSA (AHI > 30/h) had 2.25 times increased mortality — due largely to stroke and HF, but not to IHD.21 No excess mortality was seen in severe OSA treated with CPAP, or in less severe OSA.
Observational studies suggest that CPAP improves survival in severe OSA, although formal long-term RCTs are needed. The SAVE trial (ANZCTR 12608000409370; NCT00738179), instigated by the Adelaide Institute for Sleep Health, is currently underway. The trial aims to randomly allocate 2500 high CVD-risk patients with OSA to either CPAP or no CPAP, with a primary end point of time to cardiovascular event or death (results are expected in 2016). Several other large outcome-based trials are also underway, including a Spanish trial (NCT01335087) of CPAP treatment of OSA in patients with acute coronary artery syndromes. These and other studies will provide valuable clarification about whether OSA is a reversible cardiovascular risk factor. In addition, newer variants of positive airway pressure, such as adaptive servoventilation, are being tested in patients with sleep-disordered breathing and HF (NCT01164592 and NCT01128816), and we also await the results of these large multinational trials.
Provenance: Commissioned by supplement editors; externally peer reviewed.
- Garun S Hamilton1
- Matthew T Naughton2
- 1 Monash Medical Centre, Melbourne, VIC.
- 2 Allergy, Immunology and Respiratory Medicine, Alfred Hospital, Melbourne, VIC.
Matthew Naughton has been a recipient of research funding from manufacturers of CPAP equipment to undertake investigator-directed research. Garun Hamilton has been a recipient of research funding and equipment from manufacturers of CPAP equipment (Compumedics, ResMed and Philips) to undertake both investigator- and industry-directed research.
- 1. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010; 90: 47-112.
- 2. Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001; 163: 19-25.
- 3. O’Connor GT, Caffo B, Newman AB, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med 2009 15; 179: 1159-1164.
- 4. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342: 1378-1384.
- 5. Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med 2000; 160: 2289-2295.
- 6. Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 2007; 50: 417-423.
- 7. Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep 2004; 27: 934-941.
- 8. West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax 2006; 61: 945-950.
- 9. Foster GD, Sanders MH, Millman R, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 2009; 32: 1017-1019.
- 10. Iftikhar IH, Hays ER, Iverson MA, et al. Effect of oral appliances on blood pressure in obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med 2013; 9: 165-174.
- 11. Iftikhar IH, Khan MF, Das A, Magalang UJ. Meta-analysis: continuous positive airway pressure improves insulin resistance in patients with sleep apnea without diabetes. Ann Am Thorac Soc 2013; 10: 115-120.
- 12. Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med 2002; 166: 159-165.
- 13. Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet 1990; 336: 261-264.
- 14. Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173: 910-916.
- 15. Chami HA, Devereux RB, Gottdiener JS, et al. Left ventricular morphology and systolic function in sleep-disordered breathing: the Sleep Heart Health Study. Circulation 2008; 117: 2599-2607.
- 16. Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation 2010; 122: 352-360.
- 17. Mansfield DR, Gollogly NC, Kaye DM, et al. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004; 169: 361-366.
- 18. Loke YK, Brown JW, Kwok CS, et al. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2012; 5: 720-728.
- 19. Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182: 269-277.
- 20. Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLOS Med 2009; 6: e1000132.
- 21. Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J 2005; 25: 514-520.
- 22. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284: 3015-3021.
- 23. Tuomilehto HP, Seppä JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med 2009; 179: 320-327.
- 24. Johansson K, Neovius M, Lagerros YT, et al. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ 2009; 339: b4609.
- 25. Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009; 169: 1619-1626.
- 26. Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012; 308: 1142-1149.
- 27. Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19: 2271-2277.
- 28. Pépin JL, Tamisier R, Barone-Rochette G, et al. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med 2010; 182: 954-960.
- 29. Lévy P, McNicholas WT. Sleep apnoea and hypertension: time for recommendations. Eur Respir J 2013; 41: 505-506.
- 30. West SD, Nicoll DJ, Wallace TM, et al. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 2007; 62: 969-974.
- 31. Chasens ER, Strollo PJ Jr. Treatment of obstructive sleep apnea on insulin resistance: not an “anti-sugar pill”. Ann Am Thorac Soc 2013; 10: 150-151.
- 32. Munoz R, Duran-Cantolla J, Martínez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke 2006; 37: 2317-2321.
- 33. Shanoudy H, Soliman A, Raggi P, et al. Prevalence of patent foramen ovale and its contribution to hypoxemia in patients with obstructive sleep apnea. Chest 1998; 113: 91-96.
- 34. Parra O, Sánchez-Armengol A, Bonnin M, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J 2011; 37: 1128-1136.
- 35. Stevenson IH, Teichtahl H, Cunnington D, et al. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J 2008; 29: 1662-1669.
- 36. Ryan CM, Usui K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax 2005; 60: 781-785.
- 37. Craig S, Pepperell JC, Kohler M, et al. Continuous positive airway pressure treatment for obstructive sleep apnoea reduces resting heart rate but does not affect dysrhythmias: a randomised controlled trial. J Sleep Res 2009; 18: 329-336.
- 38. Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107: 2589-2594.
- 39. Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352: 1206-1214.
- 40. Pinna GD, Maestri R, Mortara A, et al. Long-term time-course of nocturnal breathing disorders in heart failure patients. Eur Respir J 2010; 35: 361-367.
- 41. Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail 2009; 15: 279-285.
- 42. Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49: 1625-1631.





Summary
The cardiovascular risk from moderate OSA (AHI, 15–30/h) is uncertain, particularly if the oxygen desaturation index is low, although the data suggest an increased risk for stroke (particularly in men). There is no evidence of increased cardiovascular risk from mild OSA (AHI < 15/h). In the elderly, the cardiovascular risks of OSA are uncertain, although there is a likelihood of increased risk of stroke. Current, ongoing randomised controlled trials will inform whether OSA is a reversible cardiovascular risk factor within the next 5 years.
Patients with cardiovascular disease, stroke, diabetes, obesity or poorly controlled hypertension are at high risk of OSA and should be questioned for symptoms of OSA, which, if present, may warrant further investigation and treatment.
Weight loss has an unpredictable effect on OSA severity, but is independently beneficial for symptoms and metabolic health in OSA patients and is recommended for all overweight and obese OSA patients.