A vast body of literature in ethics, law and medicine addresses informed consent — the process by which patients make decisions about participation in medical treatment and research through learning the benefits, risks and options.1 Most of this scholarship is normative.2 Although empirical research on the topic has grown in the last 20 years,3,4 there remains remarkably little empirical information on how the consent process actually functions (and malfunctions) in clinical practice.3,5
The human research ethics committee at the University of Melbourne approved the study.
We established definitions for claims, complaints and cases (Box 1). Our study definition of what constituted a dispute over informed consent (a “case”) was patient centred.8 We did not seek to evaluate the reasonableness or legitimacy of patients’ allegations, with one exception: if a patient alleged a failure to warn of the risks of an outcome that occurred, and there was strong clinical evidence in the case file that the outcome had not in fact occurred, we excluded the case.
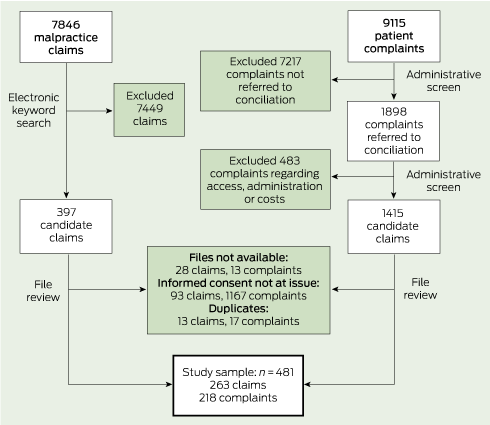
From 7846 claims, we identified 397 candidate cases through searching for keywords (eg, informed, consent, warn, risks, explain, disclose) in free-text précis of the allegations (two to five sentences written by claim managers). We then examined the full claim file on each of these to determine whether it met our case definition (Box 2). About 20% of complaints received by the HSC cannot be resolved easily by the parties involved and are referred to a dispute resolution process. These “conciliated” complaints were our focus. After ruling out conciliated complaints in several categories (access, administration, cost) that were extremely unlikely to have involved consent issues, three physician reviewers examined the hard-copy files associated with the rest (1415) to determine which met our case definition (Box 2).
To test the reliability of the review, a random subsample of cases consisting of about 10% (27) of the claims and 10% (23) of the complaints was re-reviewed by the second reviewer, who was blinded to the first review. We report κ scores9 for the primary allegation type and all allegation types because they are the only variables that required implicit judgements by the reviewers.
We calculated the frequency of informed consent disputes by specialty using rates and rate ratios. Complaint rates are the total number of consent-related complaints over the study period, divided by the sum of annual counts of registered doctors in Victoria over the study period (“doctor-years”).10 Complaint rate ratios were calculated by dividing the complaint rate for each specialty by the complaint rate for a reference group (general practitioners). Differences between rate ratios for specialties were calculated using x2 tests.
All analyses were conducted using Stata SE, version 10.0 (Stata Corp, College Station, Tex, USA).
Sixty-nine per cent of cases involved female patients (Box 3). Three-quarters of the incidents that prompted cases occurred in privately owned health care facilities and nearly two-thirds occurred in consulting rooms.
More than half (57%) of the cases were against surgeons (Box 4). Four surgical subspecialties — plastic, general, orthopaedic and ophthalmic surgery — accounted for 81% of all cases against surgeons. Obstetrics–gynaecology (14%) and general practice (11%) were the other prevalent specialties. Collectively, surgeons, obstetrician-gynaecologists and GPs were involved in 82% of cases.
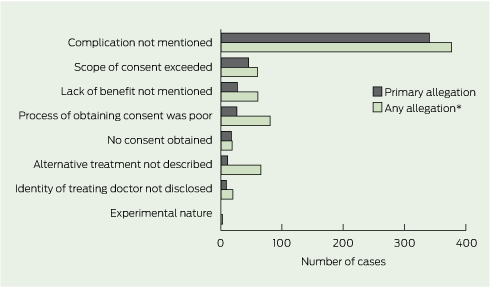
In 71% of cases, the primary allegation concerned a complication of treatment that had not been mentioned or fully understood, and then materialised (Box 5). The next most common types of primary allegations were that the scope of the consent had been exceeded (10% of cases), the risk that the procedure would confer no benefit (as opposed to harm) had not been mentioned (6%), and the process by which consent was obtained was unsatisfactory (6%). Process allegations involved situations in which patients felt rushed, pressured to proceed, or regarded the language used as incomprehensible. No case had as its primary allegation that there were problems with consent to experimental treatments or research protocols.
The disputes arose in relation to a wide array of treatments; however, clustering was evident. Thirty specific treatments accounted for 71% of cases (Box 6). There was a heavy orientation toward operations: 92% (442/481) of cases involved surgical procedures and 16% (77/481) involved cosmetic procedures. Five types of treatments — procedures involving reproductive organs (57/481; 12% of cases), procedures involving facial features excluding eyes (57/481; 12%), prescription medications (40/481; 8%), eye surgery (36/481; 7%) and breast surgery (32/481; 7%) — accounted for 46% of all cases.
To the best of our knowledge, this is the first study to investigate in a real-world setting what happens when informed consent goes poorly from the patient’s perspective — poorly enough to prompt complaints or litigation. Unlike studies that have probed patients’ views on what they want or hope to be told before treatment,11,12 we profiled situations in which patients “voted with their feet” in proclaiming unmet expectations. Improved understanding of these situations helps to spotlight facets of care in which there is a gap between what doctors do and what patients want; it may also be instructive for clinicians who are eager to avoid the vicissitudes of medicolegal processes.
In our sample, the predominance of cases alleging undisclosed risks related to surgery is striking. High-profile court battles over informed consent in the United States,13 Australia,14 Malaysia,15 New Zealand,16 Ireland,17 and Canada18 have centred on precisely this scenario.19 Concerns about surgical risks not properly explained appear to be the heartland of contemporary disputes between patients and doctors over consent, at least in Australia. By contrast, other types of breakdowns in the consent process that have attracted intense scholarly attention and debate (eg, failure to canvass alternative treatments, patient competence) appear to be infrequent triggers of formal disputes, and still others (eg, consent to services rendered as part of research), however interesting from an ethical perspective, are exotic in medicolegal fora.
Our study has several limitations. First, we describe perceived problems with the consent process that spark claims and complaints against doctors and health care institutions, but our sample may be unrepresentative of broader quality problems in this area because they are refracted through the lens of patients’ claiming behaviour.6,20 Second, we were constrained by the information set available in claim and complaint files. Finally, we took cases at face value and did not seek to make objective determinations about their legitimacy, although whether this is a true limitation is questionable in light of the study objectives.
In setting legal standards for informed consent, courts in many countries14-18 have moved towards paying deference to what reasonable patients would want to know before choosing a treatment course. Our study sheds light on patient preferences in a legal context. Consistent with the quality improvement mantra that “every defect is a treasure,”21 further research into disputes over informed consent and other preventable breakdowns in care is warranted. It has dual potential to inform risk management strategies and to guide efforts to improve the way clinicians and patients communicate about treatment choices.
1 Definitions
Case: a claim or complaint in which a patient (or patient representative) alleged that —
Received 30 March 2011, accepted 29 June 2011
- Andrew J Gogos1
- Richard B Clark2
- Marie M Bismark3
- Russell L Gruen4
- David M Studdert3,5
- 1 Royal North Shore Hospital, Sydney, NSW.
- 2 Avant Mutual Group Limited, Melbourne, VIC.
- 3 Melbourne School of Population Health, University of Melbourne, Melbourne, VIC.
- 4 Department of Surgery, The Alfred Hospital, Monash University, Melbourne, VIC.
- 5 Melbourne Law School, University of Melbourne, Melbourne, VIC.
This study was supported by a Linkage Grant from the Australian Research Council (LP0989178), with partner contributions from the Office of the Health Services Commissioner of Victoria, the Victorian Department of Health, and Avant Mutual Group Limited. David Studdert was supported by a Federation Fellowship from the Australian Research Council. Russell Gruen was supported by a Career Development Award from the National Health and Medical Research Council.
No relevant disclosures.
- 1. Berg JW, Appelbaum PS, Lidz CW, Parker LS. Informed consent: legal theory and clinical practice. 2nd ed. New York: Oxford University Press, 2001.
- 2. Dolgin JL. The legal development of the informed consent doctrine: past and present. Camb Q Healthc Ethics 2010; 19: 97-109.
- 3. Sugarman J, McCrory DC, Powell D, et al. Empirical research on informed consent. An annotated bibliography. Hastings Cent Rep 1999; 29 (1): S1-S42.
- 4. Meisel A, Roth LH. Toward an informed discussion of informed consent: a review and critique of the empirical studies. Ariz Law Rev 1983; 25: 265-346.
- 5. Leclercq WKG, Keulers BJ, Scheltinga MRM, et al. A review of surgical informed consent: past, present, and future. A quest to help patients make better decisions. World J Surg 2010; 34: 1406-1415.
- 6. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med 2006; 354: 2024-2033.
- 7. Weiler PC, Hiatt HH, Newhouse JP, et al. A measure of malpractice: medical injury, malpractice litigation, and patient compensation. Cambridge, Mass: Harvard University Press, 1993.
- 8. Krumholz HM. Informed consent to promote patient-centred care. JAMA 2010; 303: 1190-1191.
- 9. Landis J, Koch G. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159-174.
- 10. Australian Institute of Health and Welfare. Medical labour force [for the years 2002–2008]. Canberra: AIHW. (Cat. Nos. HWL 30, 32, 39, 41, 42, 45 and AUS 131.)
- 11. Dawes PJ, Davison P: Informed consent: what do patients want to know? J Royal Soc Med 1994; 87: 149-152.
- 12. Newton-Howes PA, Bedford ND, Dobbs BR, Frizelle FA. Informed consent: what do patient want to know? N Z Med J 1998; 111: 340–342.
- 13. Canterbury v Spence 464 F.2d 772 (DC Cir 1972).
- 14. Rogers v Whitaker (1992) 175 CLR 479.
- 15. Hong Chuan Lay v Dr Eddie Soo Fook Mun [1998] 5 CLJ 251.
- 16. B v Medical Council (High Court, Auckland HC11/1996, 8 July 1996).
- 17. Geoghegan v Harris [2000] 3 IR 536.
- 18. Reibl v Hughes [1980] 2 SCR 880.
- 19. Studdert DM, Mello MM, Levy MK, et al. Geographic variation in informed consent law: two standards for disclosure of treatment risks. J Emp Leg Stud 2007; 4: 103-124.
- 20. Studdert DM, Thomas EJ, Burstin H, et al. Negligent care and malpractice claiming behavior in Utah and Colorado. Med Care 2000; 38: 250-260.
- 21. Berwick DM. Escape fire. Designs for the future of health care. San Francisco: Jossey-Bass, 2004.





Abstract
Objective: To describe the frequency, characteristics, and outcomes of medicolegal disputes over informed consent.
Design and setting: Retrospective review and analysis of negligence claims against doctors insured by Avant Mutual Group Limited and complaints lodged with the Office of the Health Services Commissioner of Victoria that alleged failures in the informed consent process and were adjudicated between 1 January 2002 and 31 December 2008.
Main outcome measures: Case frequency (by medical specialty), type of allegation, type of treatment.
Results: A total of 481 cases alleged deficiencies in the informed consent process (218 of 1898 conciliated complaints [11.5%]; 263 of 7846 negligence claims [3.4%]). 57% of these cases were against surgeons. Plastic surgeons experienced dispute rates that were more than twice those of any other specialty or subspecialty group. 92% of cases (442/481) involved surgical procedures and 16% (77/481) involved cosmetic procedures. The primary allegation in 71% of cases was that the clinician failed to mention or properly explain risks of complications. Five treatment types — procedures on reproductive organs (12% of cases), procedures on facial features excluding eyes (12%), prescription medications (8%), eye surgery (7%) and breast surgery (7%) — accounted for 46% of all cases.
Conclusions: The typical dispute over informed consent involves an operation, often cosmetic, and allegations that a particular complication was not properly disclosed. With Australian courts now looking to patient preferences in setting legal standards of care for risk disclosure, medicolegal disputes provide valuable insights for targeting both quality improvement efforts and risk management activities.