The hospital environment can be a source of transmission of multiresistant organisms (MROs). Hospital infection control policies attempt to minimise cross-transmission of MROs, which include methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Enterobacteriaceae harbouring transmissible extended-spectrum β-lactamases (ESBLs) and metallo-β-lactamases (MBLs). Surfaces such as keyboards,1,2 stethoscopes,3-6 ties,7-11 lanyards12 and tourniquets1,13-15 have the potential to act as fomites and can harbour pathogenic microorganisms.
Reusable venesection tourniquets are often used consecutively on multiple patients without disinfection between uses. Current Australian health care guidelines suggest cleaning of these non-critical items with a neutral detergent on a regular basis.16 However, the required frequency of cleaning is not specified, nor whether this would prevent transmission of MROs to patients. Previous studies have indicated varying rates of MRO colonisation of reusable tourniquets, and differ based on the sensitivity of the culture method used.17 We performed this study to determine the prevalence of MRO colonisation of reusable venesection tourniquets in a Sydney teaching hospital using a sensitive enrichment method.
Tourniquet collection data are summarised in Box 1. The majority of tourniquets were collected from areas where they are frequently used, such as the blood collection unit (n = 7), and from general medical and surgical wards. Bacteria were isolated from tourniquets collected in every week of the study period. The overall bacterial colonisation rate of the 100 tourniquets was 78%. There was no bacterial growth from 22 tourniquets, and 17 grew only environmental organisms or bacteria of low pathogenic potential (coagulase-negative staphylococci and/or Bacillus spp).
Microbial colonisation data are summarised in Box 2. Many tourniquets were colonised with more than one organism. Ten grew potentially significant gram-positive organisms (methicillin-sensitive Staphylococcus aureus or Enterococcus spp), and 38 grew potentially significant gram-negative organisms (Pseudomonas spp and/or Enterobacteriaceae).
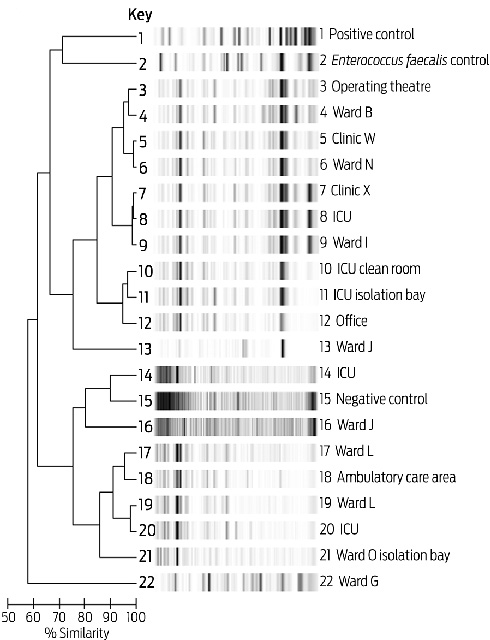
MROs were found on 25 tourniquets; however, three had been collected from MRO isolation rooms. An IMP-4 MBL-positive Enterobacter cloacae and an ESBL-positive E. cloacae were each isolated from a single tourniquet. MRSA was isolated from 14 tourniquets. VRE was isolated from 19 tourniquets: vanB-positive Enterococcus faecium from 18, and vanA-positive Enterococcus faecalis from one. Nine tourniquets isolated both MRSA and VRE, and 24 grew one or the other of these. Typing of the 18 vanB-positive isolates demonstrated five VRE clusters (Box 3). There was no apparent association between clusters of enterococci and hospital location.
Six of nine tourniquets collected from the ICU throughout the study period grew at least one MRO, although two had been used on patients known to be colonised with MRSA. MROs were isolated from tourniquets collected in most weeks of the study period (Box 1) from various hospital locations, including general wards, the ICU, burns unit, operating theatre anaesthetic bay, and the blood collection unit. The ICU had the highest rate of MRO colonisation (67% [6/9] v 23% [15/64] in wards and 13% [3/23] in ambulatory care areas).
It is estimated that around 6% of hospitalised patients will acquire an infection during their admission, leading to increased length of stay, further treatment and higher overall cost.18 To what extent tourniquets contribute to colonisation, and possibly bacteraemia, is uncertain. MRO colonisation of tourniquets may reflect the burden of MROs in the wider hospital environment and provide a measurable index of the level and quality of hospital environmental hygiene. Tourniquets may have higher potential for MRO transfer than other fomites as they are applied under pressure against the patient’s skin. They are also placed in close proximity to vascular access sites, and any skin colonisation could lead to preventable complications or health care-associated infections, such as phlebitis or cannula site infections. It is untenable that patients are exposed to potentially virulent pathogens on reused equipment.
While disposable tourniquets are readily available, their use is not universal due to perceived difficulties in application and patient discomfort. However, a study found that 85% of patients found disposable tourniquets at least as good as reusable tourniquets, and 95% of doctors found them as easy to use.19 With adequate training provided, and at a cost of about 50 cents per unit (BD, Sydney, NSW), disposable tourniquets are a viable alternative for preventing acquisition of MROs in the hospital environment. However, there is currently no supporting evidence that introducing disposable tourniquets reduces hospital MRO acquisition rates. Moreover, such a measure should be one element of a bundle of infection control measures implemented to improve hospital environmental hygiene, and hence it may be difficult to measure its contribution to reduced MRO rates.
While previous studies have demonstrated MRSA colonisation rates ranging between 0 and 42%,16 none have reported rates of VRE colonisation. We also identified colonisation by multiresistant gram-negative organisms with transmissible β-lactamase enzymes, including IMP-4. The presence of such enzymes can result in infections that are virtually untreatable with available antibiotics. These have previously been shown to be transmitted readily throughout the hospital environment.20
MROs may remain viable in the environment for a long time, as demonstrated by an MRO-colonised tourniquet (collected from an office) that had not been used for several months. When tourniquets are carried from ward to ward by hospital staff and used repeatedly, they may become a “sleeper” mechanism for unrecognised hospital MRO transmission. Of concern were the nine tourniquets that were colonised with both MRSA and VRE. This probably reflects a baseline prevalence of co-colonisation of 20%.21
A limitation of our study is that data on tourniquet use could not be collected. Previous studies have surveyed health care personnel about hand hygiene practices and glove and tourniquet use.22 The tourniquets sampled in our study were shared among multiple users and may have been used in many different hospital wards. However, this reflects the hospital’s day-to-day practice of tourniquet use. There was no way of tracking how often the tourniquet had been used, or where MRO acquisition had occurred. A British study demonstrated that contamination of tourniquets could be attributed to the user’s hands rather than the patient’s skin.23 We hypothesise that MRO colonisation of tourniquets can also be acquired from the surrounding hospital environment. The random hospital locations of the VRE clusters we recovered lends some anecdotal support to this hypothesis. A study examining colonisation of surfaces where tourniquets are stored may resolve this issue.
Previous studies16 have determined the limit of detection and performed semi-quantitative bacterial counts for MROs.23 Our study used a broth enrichment method, which may have increased sensitivity compared with methods used in previous studies, and we felt it was sufficient to demonstrate viability of bacteria from tourniquets using this method.
1 Tourniquets sampled from each location, by most clinically significant category of bacteria isolated
Received 23 March 2011, accepted 29 June 2011
- Angie N Pinto1
- Thuy Phan2
- Gabriela Sala3
- Elaine Y L Cheong4
- Steven Siarakas5
- Thomas Gottlieb6
- Department of Microbiology and Infectious Diseases, Concord Repatriation General Hospital, Sydney, NSW.
We thank Dr Amrita Ronnachit for assistance with tourniquet collection, and Dr Tony Pavic for assistance with VRE typing.
No relevant disclosures.
- 1. Fellowes C, Kerstein R, Clark J, Azadian BS. MRSA on tourniquets and keyboards. J Hosp Infect 2006; 64: 86-88.
- 2. Simmons N. Computer keyboards and the spread of MRSA. J Hosp Infect 2006; 64: 88.
- 3. Marinella MA, Pierson C, Chenoweth C. The stethoscope. A potential source of nosocomial infection? Arch Intern Med 1997; 157: 786-790.
- 4. Bernard L, Kereveur A, Durand D, et al. Bacterial contamination of hospital physicians’ stethoscopes. Infect Control Hosp Epidemiol 1999; 20: 626-628.
- 5. Varghese D, Patel H. Hand washing. Stethoscopes and white coats are sources of nosocomial infection. BMJ 1999; 319: 519.
- 6. Kennedy KJ, Dreimanis DE, Beckingham WD, Bowden FJ. Staphylococcus aureus and stethoscopes [letter]. Med J Aust 2003; 178: 468. <MJA full text>
- 7. Dixon M. Neck ties as vectors for nosocomial infection. Intensive Care Med 2000; 26: 250.
- 8. Ditchburn I. Should doctors wear ties? J Hosp Infect 2006; 63: 227-228.
- 9. Day M. Doctors are told to ditch “disease spreading” neckties [news]. BMJ 2006; 332: 442.
- 10. Biljan MM, Hart CA, Sunderland D, et al. Multicentre randomised double blind crossover trial on contamination of conventional ties and bow ties in routine obstetric and gynaecological practice. BMJ 1993; 307: 1582-1584.
- 11. Steinlechner C, Wilding G, Cumberland N. Microbes on ties: do they correlate with wound infection? Bull R Coll Surg Engl 2002; 84: 307-309.
- 12. Kotsanas D, Scott C, Gillespie EE, et al. What’s hanging around your neck? Pathogenic bacteria on identity badges and lanyards. Med J Aust 2008; 188: 5-8. <MJA full text>
- 13. Golder M, Chan CLH, O’Shea S, et al. Potential risk of cross-infection during peripheral-venous access by contamination of tourniquets. Lancet 2000; 355: 44.
- 14. Berman DS, Schaefler S, Simberkoff MS, Rahal JJ. Tourniquets and nosocomial methicillin-resistant Staphylococcus aureus infections. N Engl J Med 1986; 315: 514-515.
- 15. Ahmed SM, Ahmad R, Case R, Spencer RF. A study of microbial colonisation of orthopaedic tourniquets. Ann R Coll Surg Engl 2009; 91: 131-134.
- 16. National Health and Medical Research Council. Australian guidelines for the prevention and control of infection in healthcare. Canberra: NHMRC, 2010. http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cd33_complete.pdf (accessed Jul 2011).
- 17. Hensley DM, Krauland KJ, McGlasson DL. Acinetobacter baumannii and MRSA contamination on reusable phlebotomy tourniquets. Clin Lab Sci 2010; 23: 151-156.
- 18. Spelman DW. 2: Hospital-acquired infections [MJA Practice Essentials]. Med J Aust 2002; 176: 286-291. <MJA full text>
- 19. Kerstein RL, Fellowes C. Novel fit for purpose single use tourniquet: best of both worlds. J Med Eng Technol 2009; 33: 475-480.
- 20. Peleg AY, Franklin C, Bell JM, Spelman DW. Dissemination of the metallo-β-lactamase gene blaIMP-4 among gram-negative pathogens in a clinical setting in Australia. Clin Infect Dis 2005; 41: 1549-1556.
- 21. Reyes K, Malik R, Moore C, et al. Evaluation of risk factors for coinfection or cocolonization with vancomycin-resistant enterococcus and methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2010; 48: 628-630.
- 22. Sacar S, Turgut H, Kaleli I, et al. Poor hospital infection control practice in hand hygiene, glove utilization, and usage of tourniquets. Am J Infect Control 2006; 34: 606-609.
- 23. Leitch A, McCormick I, Gunn I, Gillespie T. Reducing the potential for phlebotomy tourniquets to act as a reservoir for meticillin-resistant Staphylococcus aureus. J Hosp Infect 2006; 63: 428-431.





Abstract
Objective: To determine the prevalence of multiresistant organism (MRO) colonisation of reusable venesection tourniquets.
Design and setting: A prospective study in a tertiary hospital to collect and analyse reusable venesection tourniquets for the presence of MROs — methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and extended-spectrum β-lactamase and metallo-β-lactamase-producing Enterobacteriaceae — using a sensitive enrichment method. Tourniquets were collected and tested during a 10-week period between September and November 2010.
Main outcome measure: Prevalence of MRO colonisation of tourniquets.
Results: The overall colonisation rate of 100 tourniquets randomly collected from general wards, ambulatory care areas and critical care areas was 78%. MROs were isolated from 25 tourniquets collected from a variety of hospital locations, including general wards, the intensive care unit, burns unit and anaesthetic bay. MRSA was isolated from 14 tourniquets and VRE from 19; both MRSA and VRE were isolated from nine tourniquets. There were no microorganisms isolated from 22 tourniquets.
Conclusion: Reusable tourniquets can be colonised with MROs and may be a potential source of transmission of MROs to hospitalised patients.