The concept of therapeutic equivalence is not new and has been used to promote efficient drug use,1-4 but formal and effective application of the concept is not as common as might be expected. When it has been used to reduce drug costs, it has sometimes been associated with mandatory drug substitution.3 An alternative strategy to encourage the use of cheaper therapeutically equivalent medicines has been to reimburse patients for only the cost of the cheapest alternative.2-4 Nevertheless, therapeutic equivalence offered a sound strategy for potentially saving significant medication costs at SH.
We aimed to develop an effective therapeutic equivalence program (TEP) at SH through voluntary collaboration and collective support of medical staff, especially senior medical staff. We also aimed to use the principles of disinvestment to ensure limited resources were put to their most effective use. Key elements of the program are outlined in Box 1.
Potential therapeutic classes to target were selected by:
identifying therapeutic classes of medicines that are associated with significant costs and that act via clearly defined pharmacological targets;
engaging directly with key clinical stakeholders (the medical head of unit and key specialists) to evaluate the acceptability of each potential therapeutic class for the TEP;
investigating the suitability of potential therapeutic classes for the TEP, based on published evidence; and
undertaking a preliminary financial impact assessment.
The strategies used to promote the TEP to prescribers included: posters in clinical areas; presentations and distribution of leaflets at grand rounds, unit meetings and medical intern tutorials; one-to-one or group meetings with key stakeholders and “academic detailing”;5 letters and memoranda to medical staff; and free promotional pens, on which “Think Therapeutic Equivalence” was printed. In addition, clinical pharmacists played an educational support role to reinforce the therapeutic equivalence concept.
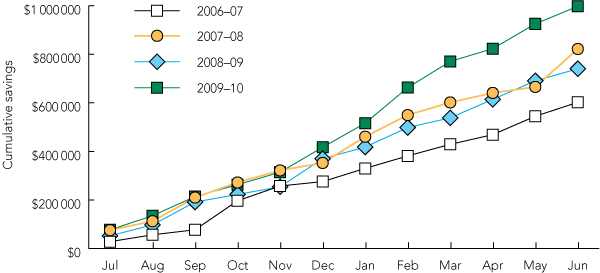
During the study period, 11 therapeutic classes were targeted (Box 2). A total of $3.16 million was saved as a result of the program, representing an average saving of $790 477 per annum (range, $602 207 to $997 418). The annual savings increased over the 4-year period (Box 3), as additional preferred medicines were included in the program. Between 2006–07 and 2009–10, TEP savings increased by 65.6%, whereas patient separations increased by 10.4% (162 720 separations in 2006–07, 179 633 separations in 2009–10).
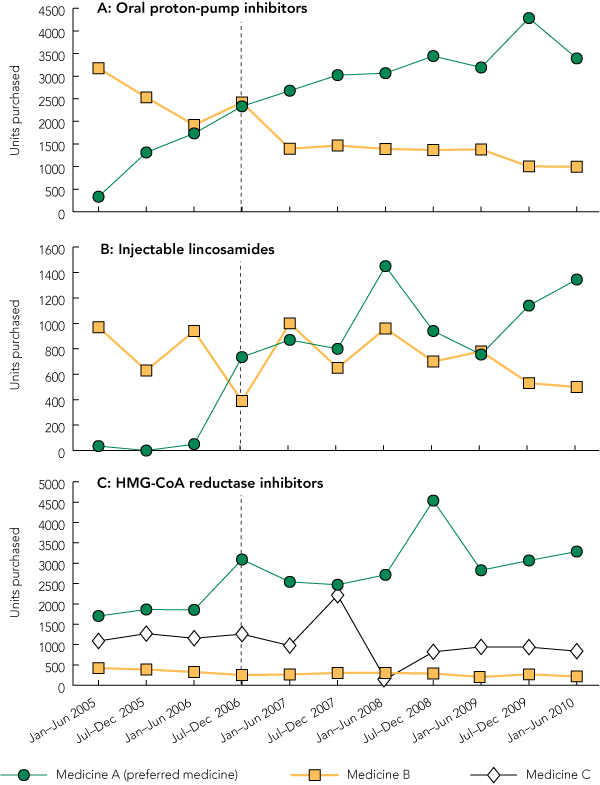
The use of all designated preferred medicines increased after their addition to the TEP, which indicated the support of medical staff for the program. However, the pattern of use varied according to how frequently the medicine was commenced in hospital and the extent of the background use of therapeutically equivalent medicines, especially for medicines that are widely used in the community. For oral proton-pump inhibitors, a clear growth in the use of the preferred medicine was seen because proton-pump inhibitors were frequently commenced in hospital (Box 4, A). However, significant background use of the alternative medicine continued because many patients were admitted to hospital on that medicine. For injectable lincosamides (which are largely used for inpatients), there was also a substantial rise in the use of the preferred medicine (Box 4, B). In this case, background use of the alternative medicine was largely due to use in paediatric patients, which was excluded from the TEP. For 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, there was background use of two alternative medicines owing to their common use in the community. Nevertheless, there was a steady increase in the use of the preferred medicine (Box 4, C) because HMG-CoA reductase inhibitors were frequently commenced in hospital.
A TEP can be considered to be a genuine disinvestment process, in which resources are diverted to their most productive use. Disinvestment is a subject of increasing interest, and discussion on how this concept should be moved forward in the Australian health care setting has begun.6 The achievements at SH indicate that therapeutic equivalence is one model of disinvestment that can succeed. However, the concept of therapeutic equivalence is a special subset of disinvestment, because it does not deal with obsolete medicine. Nevertheless it does illustrate that the process can be applied to drug therapy.
1 Key elements of the therapeutic equivalence program
Medicines in the program need to be clinically equivalent in terms of safety and efficacy
There should be no compromise to patient care
The recognised objective is to maximise potential savings
The therapeutic equivalence concept is separate from any arbitrary cost containment measures
Money saved can be used to pay for expensive medicines which might not otherwise be available, or to minimise the need to place tight restrictions on the use of expensive medications
2 Therapeutic classes targeted
Angiotensin-II receptor antagonists
Angiotensin-converting enzyme inhibitors
Oral proton-pump inhibitors
Injectable lincosamides
Penicillins
3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors
Immunosuppressants
5-HT3 antagonist antiemetics
Bisphosphonates
Glycoprotein IIb/IIIa inhibitors
Atypical antipsychotics
Provenance: Not commissioned; externally peer reviewed.
Received 12 July 2010, accepted 17 January 2011
- Ian Larmour1
- Silvana Pignataro2
- Kerryn L Barned3
- Stav Mantas4
- Melvyn G Korman5
- Southern Health, Melbourne, VIC.
The support and encouragement of the following people in making this project a reality is gratefully acknowledged: Richard Harper, Richard King, Bill Shearer, William Sievert, Wayne Ramsey; members of the High Cost Drug Working Party; staff at the Centre for Clinical Effectiveness; and medical and pharmacy staff at Southern Health.
None identified.
- 1. Larmour I, Creber E. Cultural change and drug expenditure. Aust J Hosp Pharm 1995; 25: 220-225.
- 2. Schneeweiss S. Reference drug programs: effectiveness and policy implications. Health Policy 2007; 81: 17-28.
- 3. Gumbs PD, Verschuren WM, Souverein PC, et al. Society already achieves economic benefit from generic substitution but fails to do the same for therapeutic substitution. Br J Clin Pharmacol 2007; 64: 680-685.
- 4. Schneeweiss S, Maclure M, Dormuth CR, et al. A therapeutic substitution policy for proton pump inhibitors: clinical and economic consequences. Clin Pharmacol Ther 2006; 79: 379-388.
- 5. Avorn J, Soumerai SB. Improving drug-therapy decisions through educational outreach — a randomized controlled trial of academically based detailing. N Engl J Med 1983; 308: 1457-1463.
- 6. Elshaug AG, Hiller JE, Moss JR. Exploring policy-makers’ perspectives on disinvestment from ineffective healthcare practices. Int J Technol Assess Health Care 2008; 24: 1-9.





Abstract
Objective: The development of an effective therapeutic equivalence program (TEP) through the collaborative support of medical staff, using the principles of disinvestment.
Design and setting: A TEP was introduced at Southern Health, a metropolitan health service in Melbourne, in the 2006–07 financial year. Therapeutic classes were selected for the TEP by stakeholder consensus, and a preferred medication for each class was selected on the basis of cost considerations and therapeutic equivalence. New patients were commenced on preferred medicines, but patients receiving another medicine from a therapeutic class included in the program were not automatically switched to the preferred medicine. For the first 4 years of the program, prescribing patterns were monitored, and savings achieved (due to lower prices for and increased use of preferred medicines) were calculated on a monthly basis.
Main outcome measures: Prescribing trends for preferred medicines, as a measure of acceptance of the TEP, and savings produced by the program.
Results: Over the 4-year study period, 11 therapeutic classes were targeted. The use of all preferred medicines increased once they become part of the TEP and a total of $3.16 million was saved. The annual savings increased each year, and the rate of increase was six times that of the increase in patient separations.
Conclusions: The TEP at Southern Health resulted in significant savings. It showed that, by using a collaborative and evidence-based approach, the principles of disinvestment can be applied to use of medicines.