Cancer is a life-changing diagnosis that will affect one in two men and one in three women living in Australia by the age of 85 years.1 The psychological morbidity associated with cancer is significant, with evidence suggesting that 15%–23% of cancer patients experience clinically significant anxiety and 20%–35% experience depression. The impact of these psychological disorders can be considerable, and can have major effects on relationships as well as social and occupational functioning.2 With the overall rate of death from cancer declining, the number of people living with or beyond cancer is growing; it is estimated that there are about 340 000 cancer survivors in Australia, representing about 2% of the population.3 For many, cancer becomes a chronic disease, yet relatively little is known about the psychological wellbeing of long-term cancer survivors (those surviving more than 5 years).
In contrast to the general public’s perception of cancer as an insidious and relentless disease,4 there is an emerging body of evidence suggesting that the psychosocial wellbeing of long-term cancer survivors is comparable to or only slightly lower than that of the general population.5-7 However, as most studies in this field were conducted in the United States and Europe with homogeneous samples of cancer survivors, it is unknown if these findings are indicative of the psychological wellbeing of the diverse and growing population of long-term survivors in Australia.
Our findings are based on the following instruments:
Hospital Anxiety and Depression Scale (HADS): a commonly used 14-item screening tool for anxiety and depression. Each item is rated on a four-point scale, with scores ranging from zero to 21 for each subscale. Score cut-off points classify patients’ anxiety and depression levels as within normal range, borderline or clinical.8 The HADS has demonstrated validity for detecting cases of anxiety and depression in cancer patients.9,10
-
Medical Outcomes Study Social Support Survey (MOS-SSS): a 19-item tool assessing perceived social support across four subscales (emotional/informational, tangible, affectionate and positive social interaction). Each item is rated on a five-point scale ranging from “none of the time” to “all of the time”, with a high subscale and/or overall score indicating a high level of social support. The tool has demonstrated validity in chronically ill patients.11
The Mini-Mental Adjustment to Cancer (mini-MAC) Scale: a 29-item tool assessing five cancer-specific coping strategies (helplessness–hopelessness, anxious preoccupation, fighting spirit, cognitive avoidance, and fatalism). Each item is rated on a four-point scale ranging from “definitely does not apply to me” to “definitely applies to me”, with a higher subscale score indicating stronger use of the coping strategy.12
Missing data were imputed only for those measures (MOS-SSS and mini-MAC) that recommend this procedure for handling missing data. Participants’ responses to the HADS were scored and categorised as normal (0–7), borderline (8–10) or clinical (11–21) levels of anxiety and depression.8 To explore whether any individual, disease, treatment, social support or coping characteristics predicted borderline or clinical levels of anxiety and depression, each potential predictor variable was tested for association using χ2 analyses. Variables with an association of 0.2 or less were included in the backward stepwise logistic regression model where the least significant variable was removed and the new model tested until all remaining variables in the model were significant (P < 0.05).13
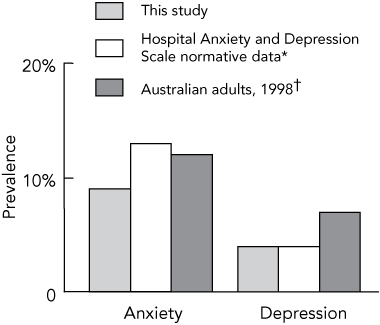
Overall, long-term cancer survivors reported low levels of anxiety (median score, 3; range, 0–20) and depression (median score, 2; range, 0–21). Only 9% of long-term survivors (76) reported clinically important levels of anxiety and 12% (101) reported borderline levels. Levels of depression were lower; only 4% (34) met the cut-off point for clinically important levels and 7% (61) met the cut-off point for borderline levels of depression. As shown in Box 1, the proportion of long-term survivors reporting clinically important levels of anxiety and depression was comparable to that in the general population.14,15
As shown in Box 2, at least half of the survivors with clinical/borderline levels of anxiety or depression reported seeing their GP in the previous 6 months, but less than 10% reported receiving specialist psychological care from a psychologist, psychiatrist, social worker or counsellor.
Contrary to popular opinion,4 the results of this study suggest that life after cancer is not all doom and gloom. By 5–6 years after a cancer diagnosis, most survivors in Australia appear to have adjusted well to their cancer experience, with levels of anxiety and depression generally comparable with those of the general Australian population.15 Although we did not have an age- and sex-matched control group to compare our survivors’ rates of anxiety and depression against, our findings are consistent with other previously published research conducted in the US and Europe.5-7 On the basis of these findings, we are currently undertaking a longitudinal study to identify the critical time-point in the lives of cancer survivors when psychological wellbeing returns to population levels.
Our study’s major strengths were its large-scale, population-based approach and the diversity of cancer survivors included. Our participation rate of 63% of all eligible survivors is comparable with or better than participation rates achieved by other Australian population-based studies.16,17 Given that our sample shows evidence of selection bias on only two of five key characteristics (age and cancer type), we are confident that our results are, by and large, representative of and generalisable to long-term cancer survivors in NSW. As it is known that younger age is associated with an increased risk of psychosocial problems,2 the under-representation of younger survivors (aged 18–39 years) in our sample is likely to have resulted in a slight underestimation of the true levels of anxiety and depression.
Nevertheless, our study suggests that there is a small and important group of long-term cancer survivors who continue to experience adverse psychological effects and need assistance. Our findings suggest that long-term cancer survivors with a history of psychological or psychiatric illness, maladaptive coping styles and poor social support should be routinely monitored as they have an increased risk of psychological problems. To date, most research seeking to identify risk factors for impaired psychosocial wellbeing has focused on demographic, disease and treatment variables.18 Our study has extended this body of research by identifying modifiable risk factors that can be targeted for intervention or prevention.
1 Prevalence of clinically important levels of anxiety and depression among long-term cancer survivors compared with the general population
 | |||||||||||||||
|
* Hospital Anxiety and Depression Scale normative data.14 † Australian Bureau of Statistics data.15 | |||||||||||||||
2 Use of psychological support services by long-term cancer survivors in the previous 6 months, by level of anxiety and depression
- Allison W Boyes1
- Afaf Girgis2
- Alison C Zucca3
- Christophe Lecathelinais4
- Centre for Health Research & Psycho-oncology, Cancer Council New South Wales, University of Newcastle and Hunter Medical Research Institute, Newcastle, NSW.
The research on which this report is based was conducted as part of the New South Wales Cancer Survival Study undertaken by the Centre for Health Research & Psycho-oncology (CHeRP). CHeRP is funded by the Cancer Council NSW and the University of Newcastle, with infrastructure funding from the Hunter Medical Research Institute. We thank Elizabeth Tracey (NSW Central Cancer Registry) for case extraction; Dianne O’Connell, Hari Grindley and Heidi Wilcox (Cancer Council New South Wales) for case recruitment; Tara Clinton and Cathy Whiteman (CHeRP) for assistance with data collection; and the cancer survivors who shared their cancer experience.
None identified.
- 1. Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer in Australia: an overview, 2006. Canberra: AIHW, 2004. (AIHW Catalogue No. CAN 32. Cancer Series No. 37.) http://www.aihw.gov.au/publications/can/ca06/ca06.pdf (accessed Feb 2009).
- 2. National Breast Cancer Centre and National Cancer Control Initiative. Clinical practice guidelines for the psychosocial care of adults with cancer. Sydney: National Breast Cancer Centre, 2003.
- 3. Australian Bureau of Statistics. National health survey: summary of results, 2004–05. Canberra: ABS, 2006. (ABS Cat. No. 4364.0.) http://www.ausstats.abs.gov.au/Ausstats/subscriber.nsf/0/3B1917236618A042CA2571 1F00185526/$File/43640_2004-05.pdf (accessed Feb 2009).
- 4. Murray M, McMillan CL. Gender differences in perceptions of cancer. J Cancer Educ 1993; 8: 53-62.
- 5. Helgeson VS, Tomich PL. Surviving cancer: a comparison of 5-year disease-free breast cancer survivors with healthy women. Psycho-oncology 2005; 14: 307-317.
- 6. Wettergren L, Björkholm M, Axdorph U, Languis-Eklöf A. Determinants of health-related quality of life in long-term survivors of Hodgkin’s lymphoma. Qual Life Res 2004; 13: 1369-1379.
- 7. Ganz PA, Rowland JH, Desmond K, et al. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. J Clin Oncol 1998; 16: 501-514.
- 8. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361-370.
- 9. Bjelland I, Dahl A, Tangen Haug T, et al. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res 2002; 52: 69-77.
- 10. Hermann C. International experiences with the Hospital Anxiety and Depression Scale — a review of validation data and clinical results. J Psychosom Res 1997; 42: 17-41.
- 11. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991; 32: 705-714.
- 12. Watson M, Law M, dos Santos M, et al. The mini-MAC: further development of the mental adjustment to cancer scale. J Psychosoc Oncol 1994; 12: 33-46.
- 13. Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons, 1989.
- 14. Crawford JR, Henry JD, Crombie C, et al. Brief report. Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol 2001; 40: 429-434.
- 15. Australian Bureau of Statistics. Mental health and wellbeing: profile of adults, Australia. Canberra: ABS, 1998. http://www.ausstats.abs.gov.au/Ausstats/subscriber.nsf/0/CA25687100069892CA25688900233CAF/$File/43260_1997.pdf (accessed Feb 2009).
- 16. Smith DP, Supramaniam R, King M, et al. Age, health, and education determine supportive care needs of men younger than 70 years with prostate cancer. J Clin Oncol 2007; 25: 2560-2566.
- 17. Girgis A, Boyes A, Sanson-Fisher RW, et al. Perceived needs of women diagnosed with breast cancer: rural versus urban location. Aust N Z J Public Health 2000; 24: 166-173.
- 18. Ayanian J, Jacobsen P. Enhancing research on cancer survivors. J Clin Oncol 2006; 24: 5149-5153.





Abstract
Objective: To assess the prevalence and predictors of anxiety and depression among a heterogeneous sample of long-term adult cancer survivors.
Design and participants: Cross-sectional survey of 863 adults diagnosed with a new histologically confirmed cancer (local or metastatic) between 1 April and 30 November 1997 and still alive in 2002, living in NSW, able to read and understand English adequately, physically and mentally capable of participating, and aware of their cancer diagnosis, who were randomly selected from the New South Wales Central Cancer Registry.
Main outcome measures: Prevalence of anxiety and depression assessed by the Hospital Anxiety and Depression Scale; and factors (patient, disease, and treatment characteristics; coping style; social support) predicting clinical or borderline levels of anxiety and depression.
Results: Levels of anxiety and depression were low; only 9% of participants reported clinically important levels of anxiety and 4% reported depression. The strongest predictive factors of borderline or clinical anxiety were previous treatment for psychological illness, maladaptive coping styles (helplessness–hopelessness, anxious preoccupation) and poor social support. Borderline or clinical depression was most strongly predicted by previous treatment for psychological illness, being an invalid pensioner, maladaptive coping style (helplessness–hopelessness) and poor positive social interaction.
Conclusions: By 5 years after diagnosis, most survivors had adjusted well to their cancer experience, with levels of anxiety and depression similar to those of the general population. Nevertheless, a small and important group of long-term survivors continue to experience adverse psychological effects and need assistance. Monitoring of psychological wellbeing and referring patients when appropriate need to be integrated into routine care for cancer survivors.