Clinical record
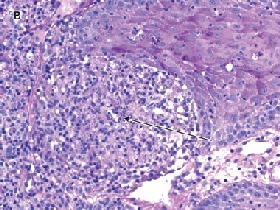
A skin biopsy from the forearm was sent for histopathology and culture (including deep fungi and mycobacteria). Histopathological examination showed an inflammatory infiltrate, most marked in the upper dermis, and poorly formed granulomas (Figure B). Mycobacterium chelonae was grown from the specimen cultures and was identified by both high-performance liquid chromatography and line probe assay at the reference laboratory at Westmead Hospital. Susceptibility testing by the agar disk diffusion technique (used to test rapidly growing mycobacteria such as M. chelonae and M. fortuitum1) showed susceptibility to clarithromycin; intermediate susceptibility to moxifloxacin, tobramycin and azithromycin; and complete resistance to all other agents, including minocycline, rifampicin, cotrimoxazole, imipenem, ciprofloxacin, cefoxitin and amikacin.
Mycobacterium chelonae is a rapidly growing, non-tuberculous mycobacterium, classified as one of the Runyon group IV mycobacteria. A ubiquitous saprophyte in the environment, it has been found in soil, water, sewage and dust particles.2,3 In humans, the organism is an uncommon cause of localised cutaneous lesions (eg, associated with surgical wounds or acupuncture) and also disseminated disease.4,5 Often the source is contamination from colonised tap water, although there have been reports of contaminated tissue-marking agents used in surgery.6,7
M. chelonae infections most commonly occur in immunosuppressed patients, such as transplant patients, in whom disease can be progressive and disseminated. In this context, there should be a high index of suspicion for M. chelonae infection.8
Tattooing carries with it several medical risks, including transmission of infectious disease due to organisms such as hepatitis B virus, HIV, Treponema pallidum, papillomavirus, and typical or atypical mycobacteria.9,10 There have been case reports of cutaneous M. tuberculosis infection following inoculation by tattooing,11 but, to our knowledge, M. chelonae infection associated with tattooing has not been previously reported.
In purely cutaneous disease, M. chelonae is introduced by trauma to the skin.12 The organism can also cause various clinical syndromes, including isolated lymphadenitis, osteomyelitis, joint infections, ocular disease and pulmonary disease. Lesions characteristically commence as red-to-violaceous subcutaneous nodules that may be painful and sometimes progress to cellulitis, abscesses or ulcers. Regional lymphadenopathy may be present,3 but constitutional symptoms are typically absent.7 Disseminated disease, usually originating from primary skin and soft tissue lesions, occurs almost exclusively in immunocompromised patients, such as those having solid organ transplants.13,14
Interestingly, in the case described here, the infection was only cutaneous and the patient was otherwise well. However, the case highlights the need to consider the possibility of systemic disease. Although there are no set guidelines for M. chelonae work-up, management of our patient warranted investigation for infectious diseases cotransmitted with tattooing as well as a chest x-ray to rule out pulmonary involvement.15
Treatment for M. chelonae infection is challenging, as the organism is resistant or only partially susceptible to many antibiotics. Based on sensitivities, and to avoid the emergence of resistance, dual treatment with clarithromycin and another antibiotic is recommended. Resistance to monotherapy with clarithromycin has been reported.8 Therapy often needs to be continued for several months, which can be difficult in terms of patient compliance.12
Because of the extensive skin surface area involved in this case, surgical excision of the affected skin was not appropriate. However, surgery may be a management option in cases in which there is abscess formation or in which drug therapy is difficult. Removal of any foreign bodies, such as iatrogenically introduced devices (eg, catheters, breast implants) is essential for management.15
Lessons from practice
Mycobacterium chelonae is a ubiquitous microorganism and an uncommon cause of infection in humans. Most infections have been reported in immunocompromised patients.
There should be a high index of suspicion for atypical pathogens in patients with skin lesions that do not respond to standard antimicrobial therapy.
Investigations should include a diagnostic skin biopsy (in formalin) sent for histopathology and a fresh specimen sent for microscopy, culture and sensitivity testing. Sensitivity testing is essential to determine the most appropriate antimicrobial therapy.
Although there is a high standard of public health regulation of tattoo parlours in Australia, cases such as these highlight the need to consider atypical pathogens as well as more common pathogens (eg, hepatitis C virus) in patients with suspected tattoo infections.
- 1. Wallace RJ, Dalovisio JR, Pankey GA. Disk diffusion testing of susceptibility of Mycobacterium fortuitum and Mycobacterium chelonei to antibacterial agents. Antimicrob Agents Chemother 1979; 16: 611-614.
- 2. Levine N, Rothschild JG. Treatment of Mycobacterium chelonae infection with controlled localized heating. J Am Acad Dermatol 1991; 24: 867-870.
- 3. Larson J, Gerlach S, Blair J, et al. Mycobacterium chelonae/abscessus infection caused by a bird bite. Infect Dis Clin Pract 2008; 16: 60-61.
- 4. Terry S, Timothy N, Zurio J, Manders E. Mycobacterium chelonae: nonhealing leg ulcers treated successfully with an oral antibiotic. J Am Board Fam Pract 2001; 14: 457-461.
- 5. Woods GL, Washington JA 2nd. Mycobacteria other than Mycobacterium tuberculosis: review of microbiologic and clinical aspects. Rev Infect Dis 1987; 9: 275-294.
- 6. Wagner D, Young LS. Nontuberculous mycobacterial infections: a clinical review. Infection 2004; 32: 257-270.
- 7. Centers for Disease Control and Prevention (CDC). Mycobacterium chelonae infections associated with face lifts — New Jersey, 2002–2003. MMWR Morb Mortal Wkly Rep 2004; 53: 192-194.
- 8. Nathan D, Singh S, Kestenbaum T, Casparian J. Cutaneous Mycobacterium chelonae in a liver transplant patient. J Am Acad Dermatol 2000; 43: 333-336.
- 9. Long GE, Rickman LS. Infectious complications of tattoos. Clin Infect Dis 1994; 18: 610-619.
- 10. Kluger N, Muller C, Gral N. Atypical mycobacteria infection following tattooing: review of an outbreak in 8 patients in a French tattoo parlor. Arch Dermatol 2008; 144: 941-942.
- 11. Wong HW, Tay YK, Sim CS. Papular eruption on a tattoo: a case of primary inoculation tuberculosis. Australas J Dermatol 2005; 46: 84-87.
- 12. Grandinetti L, Tomecki K, Pilang M, et al. Cutaneous Mycobacterium chelonae-abscessus. J Cutan Pathol 2007; 34: 88.
- 13. Patel R, Roberts G, Keating M, Paya C. Infections due to nontuberculous mycobacteria in kidney, heart and liver transplant recipients. Clin Infect Dis 1994; 19: 263-273.
- 14. Macfarlane E, Leach I, Allen B. Mycobacterium chelonae: cutaneous nodules in a diabetic patient P3-29. J Eur Acad Dermatol Venereol 2008; 17: 188.
- 15. Griffith DE, Aksamit T, Brown-Elliott B, et al. An official ATS/IDSA statement: diagnosis, treatment and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175: 367-416.






We would like to thank Associate Professor Steven Kossard of the Skin and Cancer Foundation Australia for reviewing the histology of this case and Dr Hong Foo of Westmead Hospital for her input on reviewing the mycobacterial testing.
None identified.