Mumps is an acute disease caused by infection with an RNA virus belonging to the family Paramyxoviridae and characterised by fever, swelling and tenderness of one or more salivary glands, most commonly the parotid glands.1 Orchitis is a common complication in adult males, occurring in about 20% of cases. Before the introduction of vaccination, mumps was a common cause of viral meningitis and an important cause of hearing loss in children and sterility in some men.1,2 Death from mumps is rare: in Australia, 10 deaths were reported between 1978 and 1997 and two between 1998 and 2005.3,4
The disease is highly infectious, being transmitted rapidly through droplet spread in susceptible people living in close proximity. For mumps, the basic reproduction number, R0 (ie, the number of secondary cases expected to result from an index case in a fully susceptible population) is estimated to be 10–12. This is only slightly less than the R0 for measles (15–17), another disease recognised to be highly infectious. Before the introduction of vaccination, mumps epidemics occurred every 2–5 years.5
Mumps vaccine was first available in Australia from 1981, and in combination as measles–mumps vaccine from 1983, for children at 12 months of age. From 1989, measles–mumps–rubella (MMR) vaccine was used in the national childhood immunisation schedule. A second dose of MMR vaccine was introduced for children aged 10–16 years in 1994, replacing the rubella vaccine previously given only to girls in this age group. Then, as part of the Australian Measles Control Campaign (MCC) in 1998, the second dose of MMR was moved from 10–16 years to 4 years of age, with catch-up vaccination offered to those aged between 4 and 16 years. However, school-based delivery was limited to primary schools.6 Thus, people born from 1981 onwards were eligible to receive two doses of mumps vaccine, either in high school, from 1994, or in the MCC.
After the introduction of mumps vaccination, annual reported cases of mumps declined in Australia from an estimated 59 000 in 19697 to 60 in 2002,4 but increased again recently.4,8 The aim of our study was to examine patterns of mumps epidemiology in Australia over the past decade, in the context of 1997 serosurveillance data as a proxy for vaccine coverage and the recent experience of mumps outbreaks in other developed countries.5,9-12
The seroprevalence survey was based on sera collected in 1997. The methodology of population-based serosurveillance in Australia has been previously described.13 All 52 major public and private diagnostic laboratories in Australia were invited to contribute sera, and 45 agreed to participate in 1997. The samples used for our survey had been submitted for various diagnostic tests and would otherwise have been discarded. Sera from people who were immunosuppressed, had received multiple or recent blood transfusions (within 3 months prior to our survey), or were known to be infected with HIV were excluded. Information available for each sample included a unique code number, the patient’s age and sex, and the state or territory of collection.
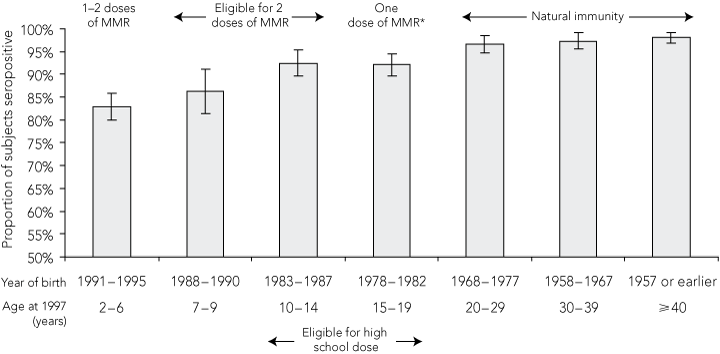
We analysed 2787 sera. The age distribution in the sample corresponded to the age distribution in the general population. The proportion of mumps-positive sera in all states and territories was high, ranging from 87.1% to 94.3%. The overall proportion of the population estimated to be seropositive for mumps was 92.1% (95% CI, 91.1%–93.1%) (Box 1).
The seropositivity rate among people born before 1978 (95.9% [1549/1615]) was significantly higher than the rate in those born in 1978 or later (86.8% [1017/1172]) (P < 0.001). Children aged 2–6 years in 1997 had the lowest proportion of seropositive results (82.9% [515/621]) (Box 2).
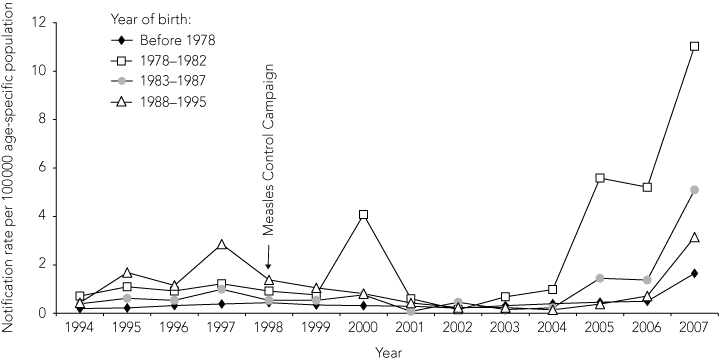
When notified cases were analysed by year of birth using broad groups corresponding to different pre-vaccination and post-vaccination eras (Box 3), the cohort born before 1978 was found to have very low and steady infection rates over the study period. In contrast, people born between 1978 and 1982 had increased notification rates, in 2000 and after 2004, while there was a progressive decline in notification rates among younger birth cohorts, particularly those born after 1987. During the period 1999–2007, the mean notification rate for the 1978–1982 birth cohort was more than threefold higher than for other birth cohorts (P < 0.001), with a significant upward trend compared with the other birth cohorts (P = 0.001).
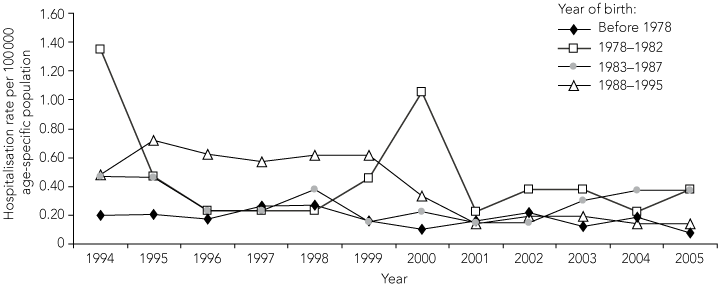
Between 1994 and 2005, 605 recorded hospitalisations were coded for mumps, including 464 (77%) with mumps as the principal diagnosis. Hospital admissions occurred in all age groups and in both sexes, with a male : female ratio of 1.2 : 1 (P = 0.03). The age distribution of hospitalised cases, using the same birth cohorts as for notifications, is shown in Box 4. Notably, for the period 1994–2005, the birth cohort of 1978–1982 had a significantly higher mean annual hospitalisation rate than pre-1978 and post-1982 birth cohorts (P = 0.04). Hospitalisation rates for cohorts born both before 1978 and after 1982 were low, and declined over time.
The cohort born before the 1970s represent the pre-vaccination era, when mumps virus transmission was widespread and seropositivity was highest (96%–98%). This is consistent with investigations of recent mumps outbreaks in other Western countries, in which age cohorts born before the vaccination era were not affected.9-12 Among those born in the 1980s, coverage with a single dose of mumps vaccine was increasing, but estimated to be only 68% among children under 5 years of age, so continuing exposure to wild virus was likely.15,16 The 1998 MCC did not target this age group as effectively as those attending primary school, and their immune profile is consistent with partial vaccination during a period of reduced exposure to wild virus transmission. It is this age cohort that has had the most noticeable recent increase in notifications, as well as hospital admissions in 2000.
Among those born after 1990, estimated coverage for at least one dose of mumps vaccine increased from 80% in 1990 to 90% in 1995,17,18 reflected by a sharp reduction in notified and hospitalised cases. Children born in the early 1990s (aged 2–6 years at the time of sampling) had the highest proportion susceptible in 1997 (17%), but would, at most, have received one dose of MMR vaccine at that time. Although seroconversion rates after a first dose of mumps vaccine under clinical trial conditions are as high as 97%,15 under field conditions vaccine effectiveness after one dose is about 88% and approaches 95% after two doses.10
In Europe, serological surveys have shown that high mumps incidence is related to low levels of population immunity in affected countries.9 However, even in countries with relatively high vaccine coverage, such as the United States11 and the United Kingdom,12 recent outbreaks have occurred, predominantly affecting young adults who have had less exposure to natural infection, as described here for Australia. Cases have also occurred among vaccinated children who had received only a single dose of MMR vaccine.10
Higher seropositivity rates in younger age groups (eligible for two doses of MMR) in Victoria compared with the rest of Australia probably reflect past high rates of MMR vaccination coverage3,18 in this state. This is consistent with higher levels of protective immunity against measles found in Victoria,19,20 which, together with lower notification rates for mumps than in the rest of Australia, suggests that higher historical MMR vaccination coverage reduced the number of cases in that state.
Our study had some limitations. First, the case definition for mumps notifications and the number of jurisdictions in which mumps was notifiable varied until 1996, when it became notifiable in all states and territories. It was recognised that a more specific case definition was needed in the context of low disease incidence,7 so the surveillance case definition for mumps was modified in 2004 to include only those with laboratory evidence of mumps or epidemiological linkage to a confirmed case.3 While changes in case definition may have affected the rates presented, notifications from 2005 to 2007 should be relatively specific, although false-positive serological results cannot be ruled out. Second, opportunistic collection of sera has the potential for bias. However, the same sample has been used to assess immunity to measles and rubella,21 and comparison between the opportunistic collection method and randomised cluster sampling found comparable estimates for measles immunity.13
Transmission of mumps virus has been considerably reduced since the introduction of vaccination. Both the waning of vaccine-acquired immunity and the accumulation of unvaccinated cohorts over time appear to have contributed to an increased susceptibility among young adults. The increased two-dose MMR coverage achieved since the 1998 MCC will reduce the risk of outbreaks among those born since 1982, although recent outbreaks have been described among two-dose recipients in the US,22 and this may have implications for Australia in later years. For now, the priority should be to target young adults, particularly those born during the late 1970s and early 1980s and now aged 25–30 years, for a second dose of MMR. In this well travelled age group, an important opportunity for giving a second dose of MMR is at the time before overseas travel. MMR vaccine is provided free of charge for all age groups in many Australian jurisdictions.
- Padmasiri E Aratchige1
- Peter B McIntyre1,2
- Helen E Quinn1,2
- Gwendolyn L Gilbert2,3
- 1 National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases, The Children’s Hospital at Westmead, Sydney, NSW.
- 2 University of Sydney, Sydney, NSW.
- 3 Centre for Infectious Diseases and Microbiology, Institute of Clinical Pathology and Medical Research, Westmead Hospital, Sydney, NSW.
The National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases (NCIRS) is supported by the Australian Government Department of Health and Ageing, NSW Health and The Children’s Hospital at Westmead. We wish to acknowledge the Australian Institute of Health and Welfare for providing data from the National Hospital Morbidity Database and the Communicable Diseases Network Australia for providing data from the National Notifiable Diseases Surveillance System. We also thank the staff of the 45 laboratories who provided the sera; laboratory staff at the Institute of Clinical Pathology and Medical Research (especially Ros Escott and Jo Backhouse); and the nurses at the NCIRS for their help in processing and testing the sera.
None identified.
- 1. Chin J. Control of communicable diseases manual. 17th ed. Washington, DC: American Public Health Association, 2000.
- 2. Leinikki P. Mumps. In: Zuckerman AJ, Banatvala JE, Pattison JR, et al, editors. Principles and practice of clinical virology. 5th ed. Chichester: Wiley, 2004: 459-466.
- 3. McIntyre PB, Gidding HF, Gilmour R, et al; National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases. Vaccine preventable diseases and vaccination coverage in Australia, 1999 to 2000. Commun Dis Intell 2002; 26 Suppl: i-xi, 1-111.
- 4. Australian Department of Health and Ageing. National Notifiable Diseases Surveillance System (NNDSS). Introduction to the National Notifiable Diseases Surveillance System. http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-surveil-nndss-nndssintro.htm (accessed Apr 2008).
- 5. Anderson RM, May RM. Immunisation and herd immunity. Lancet 1990; 335: 641-645.
- 6. National Health and Medical Research Council. The Australian immunisation handbook. 9th ed. Canberra: NHMRC, 2008.
- 7. Guy RJ, Andrews RM, Kelly HA, et al. Mumps and rubella: a year of enhanced surveillance and laboratory testing. Epidemiol Infect 2004; 132: 391-398.
- 8. Brotherton J, Wang H, Schaffer A, et al. Vaccine preventable diseases and vaccination coverage in Australia, 2003 to 2005. Commun Dis Intell 2007; 31 Suppl: S1-S152.
- 9. Nardone A, Pebody RG, van den Hof S, et al. Sero-epidemiology of mumps in western Europe. Epidemiol Infect 2003; 131(1): 691-701.
- 10. Cohen C, White JM, Savage EJ, et al. Vaccine effectiveness estimates, 2004–2005 mumps outbreak, England. Emerg Infect Dis 2007; 13: 12-17.
- 11. Centers for Disease Control (CDC). Update: multistate outbreak of mumps — United States, January 1–May 2, 2006. MMWR Morb Mortal Wkly Rep 2006; 55: 559-563.
- 12. Gupta RK, Best J, MacMahon E. Mumps and the UK epidemic 2005. BMJ 2005; 330: 1132-1135.
- 13. Kelly H, Riddell MA, Gidding HF, et al. A random cluster survey and a convenience sample give comparable estimates of immunity to vaccine preventable diseases in children of school age in Victoria, Australia. Vaccine 2002; 20: 3130-3136.
- 14. Backhouse JL, Gidding HF, McIntyre PB, Gilbert GL. Evaluation of two enzyme immunoassays for detection of immunoglobulin G antibodies to mumps virus. Clin Vaccine Immunol 2006; 13: 764-767.
- 15. Kakakios AM, Burgess MA, Bransby RD, et al. Optimal age for measles and mumps vaccination in Australia. Med J Aust 1990; 152: 472-474.
- 16. Australian Bureau of Statistics. Children’s immunisation survey, Australia, November 1983. Canberra: ABS, 1984. (ABS Cat. No. 4351.0.)
- 17. Lister S, McIntyre PB, Burgess MA, O’Brien ED. Immunisation coverage in Australian children: a systematic review 1990–1998. Commun Dis Intell 1999; 23: 145-170.
- 18. Australian Bureau of Statistics. Children’s immunisation, Australia, April 1995. Canberra: AGPS, 1995. (ABS Cat. No. 4352.0.)
- 19. Gidding HF, Gilbert GL. Measles immunity in young Australian adults. Commun Dis Intell 2001; 25: 133-136.
- 20. Kelly HA, Gidding HF, Karapanagiotidis T, et al. Residual susceptibility to measles among young adults in Victoria, Australia following a national targeted measles-mumps-rubella vaccination campaign. BMC Public Health 2007; 7: 99.
- 21. Gilbert GL, Escott RG, Gidding HF, et al. Impact of the Australian Measles Control Campaign on immunity to measles and rubella. Epidemiol Infect 2001; 127: 297-303.
- 22. Dayan GH, Quinlisk MP, Parker AA, et al. Recent resurgence of mumps in the United States. N Engl J Med 2008; 358: 1580-1589.





Abstract
Objectives: To describe the epidemiology of mumps and examine potential factors underlying the recent increase in the incidence of mumps in Australia.
Design, setting and participants: Analytical descriptive study, for all Australian states and territories, of mumps notifications (1994–2007); hospitalisations for mumps (1994–2005); and mumps seroprevalence in a nationally representative sample of 2787 subjects (1997).
Main outcome measures: Incidence of notifications and hospitalisations for mumps; seropositivity by birth cohort.
Results: Notified mumps cases increased from 60 in 2002 to 231 in 2005 and 512 in 2007. Between 1994 and 2005, there were 605 hospitalisations for mumps. Mumps seropositivity in all states and territories in 1997 was high (range, 87.1%–94.3%). The predominant age group affected by mumps shifted to adults over time: between 2005 and 2007, 41% of cases occurred among people aged 20–29 years. Cases were concentrated among the birth cohort of 1978 to 1982, who had higher rates of notifications and hospitalisations for mumps and a lower seropositivity rate (92% [95% CI, 89%–94%]) than other birth cohorts.
Conclusions: The birth cohort of 1978 to 1982 was too old to reliably receive a second dose of measles–mumps–rubella (MMR) vaccine in the 1998 Australian Measles Control Campaign and too young to have had mumps infection. Renewed efforts to maximise two-dose MMR coverage are important for prevention of mumps and measles in young adults.