Older people face a range of medication-related problems such as non-adherence to treatment regimens, use of high-risk drugs and underuse of some medicines.1 These problems can potentially lead to adverse events such as falls and reduced quality of life. Several interventions have the potential to promote quality use of medicines for older people through changing general practitioners’ behaviour. These interventions include academic detailing,2 provision of educational material,3 patient-mediated interventions,3 and audit and feedback.4 Some studies have shown medication review to be beneficial for older people living in the community,5 but others have not.6 Most studies of this kind have involved medication review by a clinical pharmacist.5,6 An alternative approach, which has not been adequately evaluated in randomised controlled trials,7 is medication review by GPs.
Our study was a cluster randomised controlled trial conducted in the Hunter Urban Division of General Practice in New South Wales in 2002. The general practice was the unit of randomisation. The intervention occurred at GP level. Outcome measurement and the unit of analysis were at patient level, with adjustment for general practice.
After extensive consultation, three drug classes were targeted.7 Recommendations were in line with those given by the National Prescribing Service at the time of the intervention. Briefly, non-steroidal anti-inflammatory drugs (NSAIDs) with low risk of gastrointestinal adverse effects (ibuprofen and diclofenac) in low doses were to be used in preference to medium- and high-risk NSAIDs8 and cyclooxygenase-2 (COX-2) inhibitors. Low-dose thiazide diuretics were promoted as first-line therapy for hypertension,9 unless there was a contraindication or specific indications for another drug. Appropriateness of long-term benzodiazepine use was targeted because of its known adverse effects and its association with falls.10
* Copies of the laminated desk-size sheets with information about the targeted drugs, Medication Risk Assessment form, Medication Review Checklist, and prescribing feedback are available from the authors on request.
The education component was conducted by a clinical pharmacist with experience in conducting medication reviews for nursing-home patients. The pharmacist visited each doctor twice and provided tailored education on how to conduct medication reviews, with emphasis on benzodiazepines, NSAIDs/COX-2 inibitors and antihypertensives. Sources of information on prescribing were provided,10,11 including one-page laminated desk-size sheets.* GPs also received feedback on the number of targeted drugs used by their patients.
Doctors received Practice Incentive Payments after completing 10 medication review checklists and were reimbursed for their time with the pharmacist.
Intervention-group participants completed a Medication Risk Assessment12 while in the waiting room. They handed the form to their doctor, who then decided whether the patient would benefit from a medication review. The form contained 31 items assessing risk factors for medication misadventure — for example, having three or more health problems, using more than four medicines, and having possible side effects such as sleep deprivation. Doctors completed a Medication Review Checklist for “at-risk” participants (Box 1).
Control-group participants also completed a Medication Risk Assessment, but the forms were collected by the researchers rather than being handed to the GP. Control doctors received no intervention except for completing a clinical audit to encourage participation in the study, which included feedback on the number of medication reviews and medication risk factors.
Participants were asked to bring to the phone all the medicines taken in the previous 7 days and to spell out the brand name and other details of each medication. All drugs were classified according to the Anatomical Therapeutic Chemical Classification 200113 by two raters who coded independently and discussed points of difference until 100% agreement on drug use was reached. The kappa (κ) statistic for agreement between coders, adjusted for overall prevalence of drug use and bias, was 0.81 (95% CI, 0.64–0.96), and the overall proportion of agreement between raters on drug use was 90%.
We constructed ordinal outcome measures for each of the targeted drugs. The scoring algorithm of a neutral score (0), success (+1) (ie, recommendation was followed), or failure (–1) (ie, recommendation was not followed) was decided prior to the intervention (details available from the authors on request). The individual drug scores were then added up to form a composite score, a six-point ordinal outcome measure. A nurse (blinded to group allocation) and the project manager independently assessed the composite score. At 12-month follow-up, a nurse and a doctor who were blinded to group allocation assessed the composite score independently. Agreement between the two assessors was 99%.
Self-reported secondary outcome measures were the proportions of participants using benzodiazepines (excluding benzodiazepines used for epilepsy), NSAIDs and thiazide diuretics; the proportion receiving a medication review; the proportion having falls;14,15 and the participants’ quality of life, as assessed by the SF-12 (version 2, standard form)16 and EQ-5D17 questionnaires.
The sample size was based on the proportions of participants using benzodiazepines, NSAIDs, and thiazide diuretics.18 The largest sample size needed to detect a difference on the three outcome measures was for NSAIDs, with 398 participants required for each group. With a power of 0.8, α = 0.05, an intra-class correlation of 0.01,19 an expected maximum cluster size of 60 participants per practice, and an inflation factor of 1.59 (because of clustering, we needed to inflate the sample size), this sample was sufficient to detect a 10% reduction from 25% to 15% in the proportion of participants using an NSAID. Allowing for 10% loss to follow-up over 4 months, we estimated that about 440 participants per group would be required.
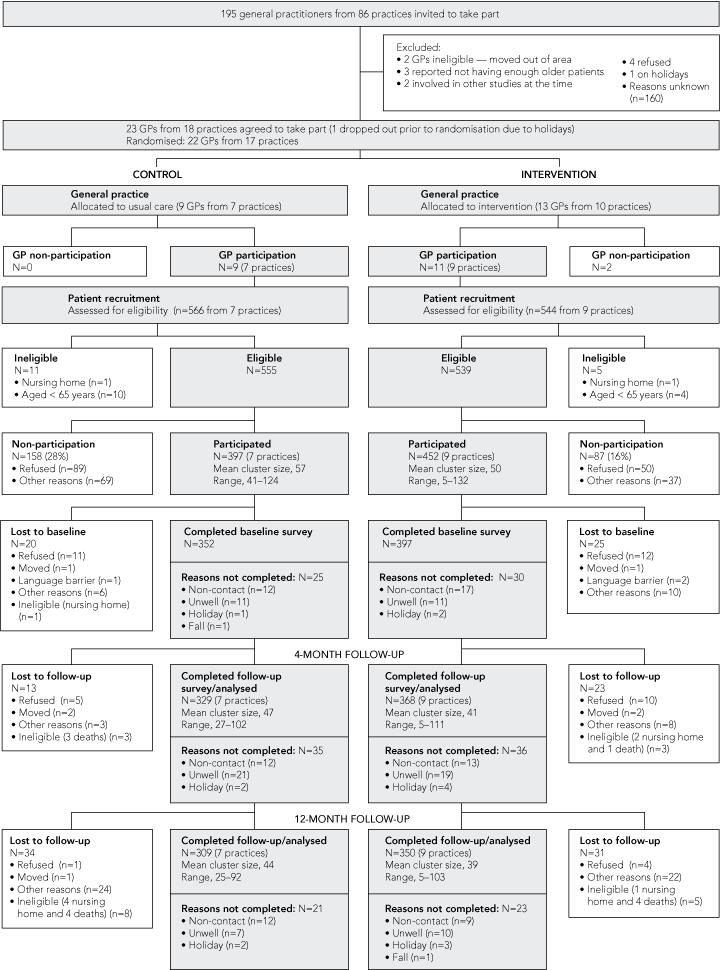
Twenty-three of 195 doctors invited to participate in our study agreed to do so (a response rate of 12%). The flow of participants is summarised in Box 2. Twenty GPs from 16 practices took part. Recruitment took place in 2002, and 12-month follow-up finished in January 2004. Participation rates were higher in the intervention group (χ2 = 2.18; P = 0.16). Reasons for loss to follow-up were similar between the two groups. There were no statistically significant differences between participants lost to follow-up and those who remained in the study in relation to sex and occurrence of previous falls by experimental group. However, a higher proportion of intervention-group participants aged ≥ 85 years were lost to follow-up (χ2 = 8.93, P = 0.03).
All doctors in the intervention group, except one who dropped out, had two academic detailing visits. The first visit took place before patient recruitment so that doctors were aware of what to look for when identifying patients for medication review. One doctor conducted reviews with all patients on the same day the Medication Risk Assessment was completed and so did not actually see all the participants’ medications (ideally, patients should have been invited back for a separate consultation to show the doctor their medications). Another doctor did not conduct any medication reviews, arguing that all patients’ medications were checked routinely. Drug-related problems identified during the reviews and actions taken to solve those problems have been published elsewhere.20
Characteristics of doctors in the two groups were generally similar, except that seven doctors in the intervention group had had over 20 years’ experience in general practice compared with only two in the control group. Participants in the intervention and control groups were reasonably similar at baseline (details available from the authors on request), except that the intervention group contained a higher proportion of women (67% versus 51%). We adjusted for this in the analysis. Participants in the intervention group had higher odds of having an improved composite score than control-group participants (OR, 1.86; 95% CI, 1.21–2.85) at 4-month follow-up, but not at 12-month follow-up (Box 3). Participants in the intervention group had lower odds of using NSAIDs than control-group participants at 4-month follow-up but not at 12 months. No significant changes were found for benzodiazepines or thiazide diuretics at 4- and 12-month follow-up.
At 4-month follow-up, crude ORs, but not adjusted ORs, showed significant differences between the groups in relation to the proportion of people having falls (Box 4). At 12-month follow-up, intervention-group participants had lower adjusted odds of having a fall, fall injury or fall requiring medical attention. The intervention produced no significant difference in quality-of-life scores after adjustment for age, sex and baseline scores (Box 5).
Our intervention, which aimed to encourage medication review by GPs, improved medicines use in the short term but not in the longer term, and the effects were modest. Prescription of NSAIDs was the only drug-use measure to show a statistically significant change. As in a similar Australian study,21 thiazide diuretic use did not increase. There was a reduction in falls in the intervention group that was sustained over a 12-month period. This was not easily explained by changes in medication use and may be a chance finding, but it raises the possibility that the intervention had other effects (see below).
The intervention may not have adequately addressed barriers to prescribing thiazides. There may be conceptual and behavioural differences between adding and reducing medications. Changes in prescribing are more likely to occur in newly diagnosed hypertensive patients and less likely to be seen in patients who have been using antihypertensive drugs without any problems. Moreover, as specialists have a substantial impact on prescribing, there may be a need to involve both GPs and specialists in efforts to improve prescribing behaviour.22
Compared with our study, Zermansky et al5 reported a large increase in medication review rates over a 1-year period during their intervention in the United Kingdom (97% of patients in the intervention group had medication reviews compared with 44% in the control group). This difference may be attributable to several factors. In the study by Zermansky et al, a pharmacist conducted medication reviews and review rates were determined from medical records, whereas we relied on self-report. Thus, medication reviews may have been under-reported in our study.
The effect of our intervention on the number of falls was more visible after 12 months than after 4 months. It may be that the higher number of reported falls over a 12-month period increased the power of the study to detect a difference. The intervention itself may have had other effects, as it addressed issues besides drug use, such as compliance, postural hypotension and doctors’ awareness of falls (eg, as a risk factor on the Medication Risk Assessment form). Alternatively, the apparent impact on falls may have been due to a type 1 error or to loss of some patients to follow-up. A higher proportion of intervention-group participants aged ≥ 85 years were lost to follow-up, which may partly explain the lower proportion of falls in the intervention group at follow-up. Also, there may have been self-report bias, with those in the intervention group being less likely to report falls (however, previous research shows that participants in intervention groups are more likely to report falls).14 Our results are supported by other studies aimed at reducing falls through medication review. However, these interventions also addressed fall-risk factors other than drugs, such as home modification (Dr Sue Carter, Director of Strategic and Clinical Service Planning, Division of Population Health, Planning and Performance, Hunter New England Area Health Service, NSW, personal communication) or exercise.23 Meredith et al,24 who solely addressed medication problems for older people, found no effect of their intervention on falls.
There was an apparent tendency for quality-of-life scores to increase over time in both groups, but adjusted ORs showed the differences to be non-significant. Similarly, a systematic review investigating the effects of pharmaceutical services on quality of life25 concluded that what appeared to be a positive trend was non-significant.
The doctors’ low response rate to the invitation to participate in our study may limit the generalisability of the findings, as it is likely that participating doctors were those with an interest in medicine-related issues in elderly patients. However, participating GPs’ demographic and practice characteristics were similar to national data. The low response rate was in line with the rate for other high-intensity intervention studies.26,27 Patient response rates were difficult to determine, as office staff did not always systematically record information about non-participants. Reasons for loss to follow-up were similar in both groups. The fact that participation rates were higher in the intervention group raises questions about the effectiveness of blinding. Furthermore, improvements were found in the control group as well, suggesting a Hawthorne effect. Our study was underpowered to detect significant differences for the proportions of benzodiazepines, NSAIDs and thiazide diuretics used between study arms due to higher loss of participants to follow-up than expected.
Our approach of recording the prevalence of use of several targeted drugs was a crude measure of appropriate prescribing. For example, it did not take into account the indications for prescribing. For all the outcome measures, we attempted to approximate participants’ comorbidity profiles by controlling for quality of life, which has been shown to be correlated with physical and mental health conditions.28
Self-reporting of medicine use may lead to bias. The reliability of self-reporting was validated by comparing self-reports with findings obtained from home visits and pharmaceutical claims data. There was high agreement between self-reported and actual drug use (> 90% for home visits and > 86% for pharmaceutical claims data).7 The composite score also showed high agreement between self-reported and actual drug use obtained from home visits (κ = 0.87; 95% CI, 0.76–0.98).
1 Main components of the Medication Review Checklist
Each section contained a list of problems to consider and potential solutions to any problems:
Awareness of number of drugs used
Compliance issues
Several specific drug categories (benzodiazepines, tricyclic antidepressants, selective serotonin reuptake inhibitors, antipsychotics, other sedatives/hypnotics/tranquilisers/ antidepressants, non-steroidal anti-inflammatory drugs, cyclooxygenase-2 inhibitors, other analgesics, diuretics, β-blockers, α-blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers, H2 antagonists, proton-pump inhibitors, prochlorperazine, metoclopramide, oral corticosteroids, sulfonylureas, metformin, other hypoglycaemics, digoxin, quinine, allopurinol, over-the-counter or complementary medicines and warfarin)
Adverse drug reactions
Recommendation section
3 Number (%) of patients reporting having had a medication review since taking part in the study and drug use at baseline and at 4- and 12-month follow-up, by experimental group (logistic regression); and comparison of composite scores at 4- and 12-month follow-up, by experimental group (polytomous logistic regression)
Due to the size of this table, please view it in the article pdf
Received 9 September 2006, accepted 30 April 2007
- Sabrina W Pit1
- Julie E Byles2
- David A Henry1
- Lucy Holt3
- Vibeke Hansen1
- Deborah A Bowman1
- 1 School of Medical Practice and Public Health, Faculty of Health, University of Newcastle, Newcastle, NSW.
- 2 Research Centre for Gender, Health and Ageing, Faculty of Health, University of Newcastle, Newcastle, NSW.
- 3 Mater Hospital, Newcastle, NSW.
Professor Jill Cockburn (deceased) is especially acknowledged. She conceived, designed, analysed and interpreted the results of our study until October 2004. We thank all the GPs (in particular Dr Parker Magin and Dr Peter Hopkins) and their patients for participating; Chris Bonner for advice and for providing the Therapeutic flags manual; Professor Tony Smith for advice; Professor Paul Glasziou for developing the grant; the Royal Australian College of General Practitioners for approving parts of the research as a clinical audit and as a continuing professional development activity; and the National Prescribing Service for approving Practice Incentive Payments. We also thank the advisory group members, interviewers and statisticians (in particular Christophe Lecathelinais and Sara Wheeles). The National Health and Medical Research Council provided funding, and Sabrina Pit received a University of Newcastle Postgraduate Scholarship as a PhD student. The funding bodies had no input into the content or preparation of this article.
None identified.
- 1. Roughead EE, Barratt JD, Gilbert AL. Medication-related problems commonly occurring in an Australian community setting. Pharmacoepidemiol Drug Saf 2004; 13: 83-87.
- 2. Soumerai SB, McLaughlin TJ, Avorn J. Improving drug prescribing in primary care: a critical analysis of the experimental literature. Milbank Q 1989; 67: 268-317.
- 3. Grimshaw JM, Thomas R, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004; 8 (6): iii-iv, 1-72.
- 4. Grimshaw JM, Shirran L, Thomas R, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care 2001; 39 (8 Suppl 2): II2-II45.
- 5. Zermansky AG, Petty DR, Raynor DK, et al. Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial. Health Technol Assess 2002; 6 (20): 1-86.
- 6. Sellors J, Kaczorowski J, Sellors C, et al. A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients. CMAJ 2003; 169: 17-22.
- 7. Pit S. Improving quality use of medicines for older people in general practice [PhD thesis]. Newcastle, NSW: University of Newcastle, 2004.
- 8. Henry D, Lim LL, Garcia Rodriguez LA, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ 1996; 312: 1563-1566.
- 9. Scottish Intercollegiate Guidelines Network. Hypertension in older people. A national clinical guideline. Edinburgh: SIGN, 2001.
- 10. Australian medicines handbook. Adelaide: Australian Medicines Handbook Pty Ltd, 2002.
- 11. Bonner C, Tett S, Minck S. Therapeutic flags. Healthy prescribing for the elderly. Brisbane: University of Queensland, 2001.
- 12. Pit S, Byles J. Prevalence of self-reported risk factors for medication misadventure among older people in general practice. J Eval Clin Pract 2007. In press.
- 13. WHO Collaborating Centre for Drug Statistics Methodology. Anatomical therapeutic chemical (ATC) classification index with defined daily doses (DDDs). Oslo: WHO Collaborating Centre for Drug Statistics Methodology, 2001.
- 14. Mackenzie L, Byles J, D’Este C. Validation of self-reported fall events in intervention studies. Clin Rehabil 2006; 20: 331-339.
- 15. Cohen I, Rogers P, Burke V, Beilin L. Predictors of medication use, compliance and symptoms of hypotension in a community-based sample of elderly men and women. J Clin Pharm Ther 1998; 23: 423-432.
- 16. Ware JE, Turner-Bowker DM, Kosinski M, Gandek B. SF-12v2: how to score version 2 of the SF-12 Health Survey. Lincoln: QualityMetric Incorporated, 2002.
- 17. Tamim H, McCusker J, Dendukuri N. Proxy reporting of quality of life using the EQ-5D. Med Care 2002; 40: 1186-1195.
- 18. Donner A, Klar N. Design and analysis of cluster randomization trials in health research. London: Arnold, 2000.
- 19. Underwood M, Barnett A, Hajioff S. Cluster randomization: a trap for the unwary. Br J Gen Pract 1998; 48: 1089-1090.
- 20. Pit S, Byles J, Cockburn J. Medication review: patient selection and general practitioner’s report of drug-related problems and actions taken in elderly Australians. J Am Geriatr Soc 2007; 55: 927-934.
- 21. Bolton G, Tipper S, Tasker J. Medication review by GPs reduces polypharmacy in the elderly: a Quality Use of Medicines program. Aust J Primary Health 2004; 10: 78-82.
- 22. Robertson J, Fryer JL, O’Connell DL, et al. The impact of specialists on prescribing by general practitioners. Med J Aust 2001; 175: 407-411.
- 23. Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med 1994; 331: 821-827.
- 24. Meredith S, Feldman P, Frey D, et al. Improving medication use in newly admitted home healthcare patients: a randomized controlled trial. J Am Geriatr Soc 2002; 50: 1484-1491.
- 25. Pickard AS, Johnson JA, Farris KB. The impact of pharmacist interventions on health-related quality of life. Ann Pharmacother 1999; 33: 1167-1172.
- 26. Kable S. An application of interactive computer programs to promote adherence to clinical practice guidelines for childhood asthma in general practice [PhD thesis]. Newcastle, NSW: University of Newcastle, 2004.
- 27. Reeve JF, Peterson GM, Rumble RH, Jaffrey R. Programme to improve the use of drugs in older people and involve general practitioners in community education. J Clin Pharm Ther 1999; 24: 289-297.
- 28. Pettit T, Livingston G, Manela M, et al. Validation and normative data of health status measures in older people: the Islington study. Int J Geriatr Psychiatry 2001; 16: 1061-1070.





Abstract
Objective: To investigate the effectiveness of an educational Quality Use of Medicines program, delivered at the level of general practice, on medicines use, falls and quality of life in people aged ≥ 65 years.
Design: Cluster randomised controlled trial conducted in 2002.
Setting: General practices in the Hunter Region, New South Wales, Australia.
Participants: Twenty general practitioners recruited 849 patients to participate in the study.
Intervention: Education (academic detailing, provision of prescribing information and feedback); medication risk assessment; facilitation of medication review; financial incentives.
Main outcome measures: Primary measures: a composite score reflecting use of benzodiazepines, non-steroidal anti-inflammatory drugs (NSAIDs) and thiazide diuretics; secondary measures: use of medication reviews, occurrence of falls, quality of life (as assessed by SF-12 and EQ-5D survey scores.
Results: Compared with the control group, participants in the intervention group had increased odds of having an improved medication use composite score (odds ratio [OR], 1.86; 95% CI, 1.21–2.85) at 4-month follow-up but not at 12 months. At 4-month follow-up, the intervention group had reduced odds of using NSAIDs (OR, 0.62; 95% CI, 0.39–0.99) and showed a non-significant reduction in use of benzodiazepines (OR, 0.51; 95% CI, 0.20–1.30) and thiazide diuretics (OR, 0.70; 95% CI, 0.48–1.01). Changes in drug use were not significant at 12-month follow-up. At 12 months, intervention-group participants had lower adjusted ORs (AORs) for having a fall (AOR, 0.61; 95% CI, 0.41–0.91), injury (AOR, 0.56; 95% CI, 0.32–0.96), and injury requiring medical attention (AOR, 0.46; 95% CI, 0.30–0.70). Quality-of-life scores were unaffected by the intervention.
Conclusion: Education and systems for medication review conducted by GPs can be used to improve use of medicines. These interventions are associated with a reduction in falls among older people, without adverse effects on quality of life.