Colorectal cancer is the most commonly reported cancer in Australia; in 2001, there were 12 619 new cases and 4686 deaths.1 A diagnosis of colorectal cancer may be associated with considerable physical and psychological morbidity,2-4 and the disease imposes a significant economic burden.5 As the Australian population ages, the incidence of colorectal cancer is expected to increase accordingly.1
The prognosis for patients diagnosed with a localised tumour is good, with 5-year survival achieved by about 90%; however, only about 7% of patients with metastatic colorectal cancer achieve this goal.6 For symptomatic patients, delay in diagnosis does not appear to be associated with more advanced disease.7-10 Diagnosis of colorectal cancer in asymptomatic patients by faecal occult blood testing (FOBT) and flexible sigmoidoscopy has been shown to improve survival.11-13
In 1997, the Australian Health Technology Advisory Committee produced a report that called for an Australian trial of colorectal cancer screening by FOBT for individuals from the age of 50.14 Subsequently, the Australian Government established the Bowel Cancer Screening Pilot Program in Mackay (Queensland), Melbourne and Adelaide, which found that screening for colorectal cancer using FOBT was feasible, acceptable and cost-effective.15 The government has now introduced a national screening program, initially for those turning 55 or 65 years of age between May 2006 and June 2008, which will be evaluated in 2008.15
The aim of our study was to systematically describe the process of colorectal cancer diagnosis in Queensland before the introduction of a population screening program, and to establish if and how diagnosis could be improved. Specifically, we wished to determine the factors associated with time to diagnosis, focusing on patients’ age, category of social advantage or disadvantage, and health insurance status.
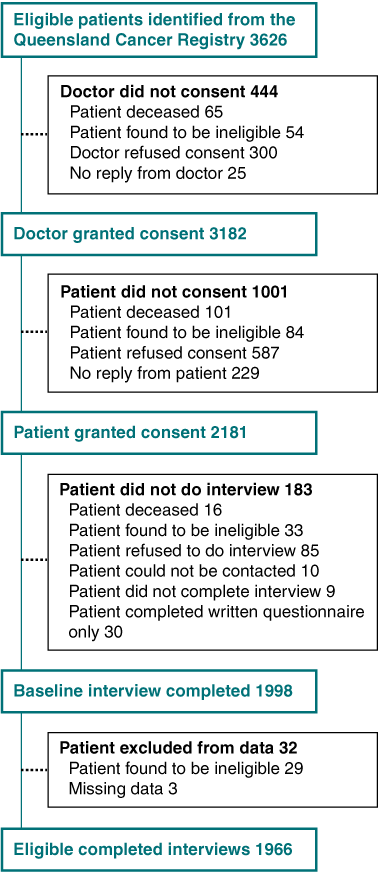
A flow diagram describing recruitment to, and participation in, the study is presented in Box 1. The treating doctors of 3626 eligible patients were approached in writing for permission to contact their patients regarding the study. Letters were re-sent to non-responding doctors 2 weeks after the initial mailing, and doctors were then telephoned weekly until an answer was received.
We calculated two measures of time to diagnosis:16 pre-presentation time — the time from first noticing a symptom or thinking something was wrong to first presenting to a doctor; and post-presentation time — the time between first presenting to a doctor and a diagnosis being made.
A total of 1966 of the 3426 eligible participants (57.4% overall response rate) completed the interview. To examine the representativeness of our sample, we compared participants with those who were eligible but not recruited to the study, across the demographic and disease-specific variables available to us. Non-participants comprised those whose doctors did not grant consent for them to be contacted about the study and those who declined to participate (Box 2). There was no difference in sex distribution between participants and non-participants. However, our sample under-represents older (70–80 years) colorectal cancer survivors, those with rectal cancer, and those with more advanced disease (χ2 test, P < 0.05 for each).
Study participants were asked to describe the symptoms that made them think something was wrong, or the other reasons that prompted them to see a doctor. These data are presented in Box 3 (multiple responses were allowed). Of the 1467 participants who reported experiencing colorectal cancer symptoms, 927 (63%) reported one symptom, 425 (29%) reported two, 96 (7%) reported three, 12 (1%) said they had four symptoms, and seven reported five or more symptoms thought to be related to colorectal cancer. Only 37 participants (2%) reported that they had first seen a doctor in order to collect FOBT results. Of these, 15 were from the Mackay region, where the Australian Government’s Bowel Cancer Screening Pilot Program was conducted during much of our study recruitment phase.
The following factors were entered into the multinomial logistic regression model for pre-presentation time: rectal bleeding, abdominal pain, change in bowel habit, site of colorectal cancer, age group, SEIFA category, and health insurance status (Box 4). Abdominal pain, change in bowel habit, site, and age group were significantly associated with the time between first noticing a symptom and seeing a doctor. Patients with abdominal pain, and those who were older, tended to have shorter pre-presentation times. For example, people who presented to a doctor within 1 week were 1.7 times more likely to experience abdominal pain than those who waited for 2 months or more. The odds of having longer pre-presentation times were greater among patients with a change in bowel habit, and those with rectal cancer. People without health insurance were more likely to have longer pre-presentation times, but this difference was not statistically significant.
For the post-presentation time model, similar variables (sex instead of site) were included (Box 5). Abdominal pain, change in bowel habit, sex, and health insurance status were significantly associated with the time between seeing a doctor and a diagnosis of colorectal cancer being made. Patients with abdominal pain were significantly more likely to have shorter post-presentation times, while women, as well as patients who experienced a change in bowel habit, were significantly more likely to have longer post-presentation times. Patients without private health insurance were significantly more likely to have a longer time to diagnosis than those who were insured.
The results of our study suggest that the majority of people diagnosed with colorectal cancer in Queensland in 2003 and 2004 were symptomatic. Fewer than 10% of the sample were diagnosed through screening or routine surveillance; only 2% of patients with colorectal cancer in Queensland were reportedly diagnosed by FOBT screening. Given this low starting level, we expect to see a large increase in colorectal cancer diagnoses as population screening is introduced.
2 Comparison of demographic and medical characteristics of study participants and non-participants
* Missing data within some categories: site (244); stage (481). † Missing data within some categories: site (5); stage (392). |
|||||||||||||||
4 Adjusted odds ratios* of predictors of pre-presentation time: results of multinomial logistic regression model
Pre-presentation time (reference, > 2 months) |
|||||||||||||||
- Brigid M Lynch1
- Peter Baade1
- Lin Fritschi2
- Barbara Leggett3
- Neville Owen4
- Kenneth Pakenham5
- Beth Newman6
- Joanne F Aitken1
- 1 Viertel Centre for Research in Cancer Control, Queensland Cancer Fund, Brisbane, QLD.
- 2 Western Australian Institute for Medical Research, Perth, WA.
- 3 Royal Brisbane and Women’s Hospital, Brisbane, QLD.
- 4 Cancer Prevention Research Centre, School of Population Health, University of Queensland, Brisbane, QLD.
- 5 Behaviour Research and Therapy Centre, School of Psychology, University of Queensland, Brisbane, QLD.
- 6 School of Public Health, Queensland University of Technology, Brisbane, QLD.
Our study was funded by the Queensland Cancer Fund.
None identified.
- 1. Australian Institute of Health and Welfare; Australasian Association of Cancer Registries. Cancer in Australia 2001. Canberra: AIHW, 2004. http://www.aihw.gov.au/publications/can/ca01/ca01.pdf (accessed Feb 2007).
- 2. Arndt V, Merx H, Stegmaier C, Brenner H. Quality of life in patients with colorectal cancer one year after diagnosis compared with the general population: a population-based survey. J Clin Oncol 2004; 22: 4829-4836.
- 3. Forsberg C, Cedermark B. Well-being, general health and coping ability: one year follow-up of patients treated for colorectal and gastric cancer. Eur J Cancer Care (Engl) 1996; 5: 209-216.
- 4. Schag C, Ganz P, Wing D, et al. Quality of life in adult survivors of lung, colon and prostate cancer. Qual Life Res 1994; 3: 127-141.
- 5. Redaelli A, Cranor C, Okano G, Reese P. Screening, prevention and socioeconomic costs associated with the treatment of colorectal cancer. Pharmacoeconomics 2003; 21: 1213-1238.
- 6. Australian Cancer Network Colorectal Cancer Guidelines Revision Committee. Guidelines for the prevention, early detection and management of colorectal cancer. Sydney: The Cancer Council Australia and Australian Cancer Network, 2005.
- 7. Gonzalez-Hermoso F, Perez-Palma J, Marchena-Gomez J, et al. Can early diagnosis of symptomatic colorectal cancer improve the prognosis? World J Surg 2004; 28: 716-720.
- 8. Khattak I, Eardley N, Rooney P. Colorectal cancer: a prospective evaluation of symptom duration and GP referral patterns in an inner city teaching hospital. Colorectal Dis 2006; 8: 518-521.
- 9. Kiran P, Glass R. Duration of symptoms and spread of colorectal cancer: a short history does not mean early disease. Ann R Coll Surg Engl 2002; 84: 381-385.
- 10. Rupassara K, Ponnusamy S, Withanage N, Milewski P. A paradox explained? Patients with delayed diagnosis of symptomatic colorectal cancer have good prognosis. Colorectal Dis 2006; 8: 423-429.
- 11. Jorgensen O, Kronborg O, Fenger C. A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut 2002; 50: 29-32.
- 12. Mandel J, Church T, Ederer F, Bond J. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 1999; 91: 434-437.
- 13. Selby J, Friedman G, Quesenberry C, Weiss N. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med 1992; 326: 653-657.
- 14. Australian Health Technology Advisory Committee. Colorectal cancer screening. Canberra: Australian Government Publishing Service, 1997: 104.
- 15. Australian Government Department of Health and Ageing. National Bowel Cancer Screening Program. About the program. http://www.cancerscreening.gov.au/internet/screening/publish ing.nsf/Content/bowel-1lp (accessed Dec 2006).
- 16. Baade P, English D, Youl P, et al. The relationship between melanoma thickness and time to diagnosis in a large population-based study. Arch Dermatol 2006; 142: 1422-1427.





Abstract
Objective: To describe the process of colorectal cancer diagnosis in Queensland, and to determine factors associated with time to diagnosis.
Design, setting and participants: Cross-sectional study of 1996 patients with colorectal cancer recruited through the Queensland Cancer Registry. Data were collected by computer-assisted telephone interview between May 2003 and August 2005.
Main outcome measures: Time to diagnosis: pre-presentation time (time from first noticing a symptom to first presenting to a doctor); and post-presentation time (time between the first presentation and diagnosis).
Results: Most patients (90%) had experienced symptoms before being diagnosed with colorectal cancer; only 2% of patients were diagnosed by faecal occult blood testing. Older participants and those who experienced abdominal pain had the shortest time from symptom onset to their first doctor consultation, while participants with a change in bowel habit, or rectal bleeding, and those without private health insurance tended to wait longer to see a doctor. Participants who experienced abdominal pain were diagnosed more quickly, whereas those who experienced a change in bowel habit, women, and those without private health insurance experienced a longer time to diagnosis.
Conclusions: The strong association between not having health insurance and longer post-presentation times is concerning. The other hypothesised predictors of time to diagnosis were not as strongly associated as we anticipated.