This article is an abbreviated version of the full guidelines, Guidelines for the prevention, detection and management of people with chronic heart failure in Australia 2006, by the National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand. This document is available at <http://www.heartfoundation.com.au>.
Australian data on the epidemiology and public health significance of chronic heart failure (CHF) are limited. Current estimates rely largely on information from large-scale population cohort studies in the United States and Europe.1 While CHF is found in 1.5%–2.0% of Australians, the overall pattern of CHF shows that its incidence and prevalence rise markedly with age.2,3 The point prevalence of CHF has been about 1% in people aged 50–59 years, 10% in people aged ≥ 65 years, and over 50% in people aged ≥ 85 years.1,4
Based on these international estimates and Australian data, about 300 000 Australians are believed to have CHF at any one time, and at least 10% of Australians aged over 65 years develop CHF.5 In addition, extrapolation of more extensive Australian data suggests that, in the year 2000, there were more than 20 000 incident hospital admissions for CHF, particularly in the elderly (≥ 70 years), and that it was associated with a total of 100 000 hospitalisations and contributed to 1.4 million days of hospital stay.5,6 From a primary care perspective, CHF engenders a complexity of issues relating to its detection and optimal management, and is one of the most common reasons for elderly patients to consult a general practitioner. A survey of 341 Australian GPs estimated that, for every 100 patients aged over 60 years seen in general practice, 11 had known CHF and two would be newly diagnosed as having CHF based on clinical features and known aetiological factors.7
Overall, chronic cardiovascular disease accounts for more than $5 billion per year in health care costs in Australia,8 and, although there are no precise data, CHF is estimated to contribute to more than $1 billion of these costs.6 The major driver of CHF-related health care costs is hospitalisation (about two-thirds of total expenditure), the major preventable cost component being recurrent hospital stays. The cost burden of CHF in Australia is also expected to increase markedly because of:
the ageing of the Australian population;
the projected increase in the number of older people with common precursors of CHF (eg, coronary heart disease and hypertension);
an increasing prevalence of obesity and metabolic syndromes;
improved survival rates for people with CHF;
a continued decline in case-fatality rates associated with acute coronary syndromes; and
improved diagnosis of CHF because of increased use of sensitive (eg, echocardiography) and point-of-care (eg, B-type natriuretic peptide [BNP]) tests.
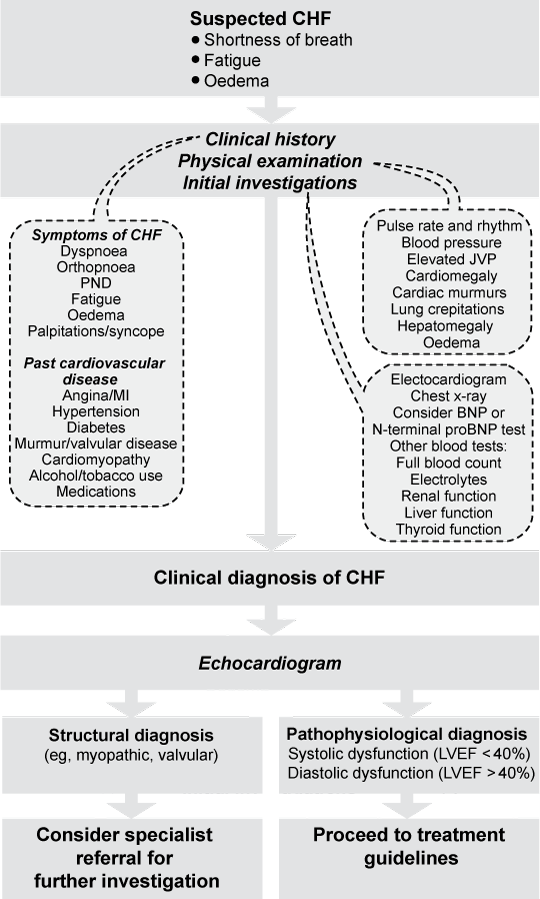
Common causes of CHF are ischaemic heart disease (present in > 50% of new cases), hypertension (about two-thirds of cases) and idiopathic dilated cardiomyopathy (around 5%–10% of cases). Systolic (impaired contractile function) and diastolic (impaired relaxation) heart failure often coexist; the distinction between them is relevant to the therapeutic approach. Diagnosis is based on well known clinical features and appropriate investigations, not only to confirm or exclude the diagnosis of CHF, but also to establish underlying causes for which particular treatment is necessary (see Box 1 for a diagnostic algorithm for CHF). In this position statement, CHF is defined as:
A complex clinical syndrome with typical symptoms (eg, dyspnoea, fatigue) that can occur at rest or on effort that is characterised by objective evidence of an underlying structural abnormality OR cardiac dysfunction that impairs the ability of the ventricle to fill with or eject blood (particularly during exercise). A diagnosis of CHF may be further strengthened by a beneficial clinical response to treatment(s) directed towards amelioration of symptoms associated with this condition.
Recommendations relating to the diagnosis of CHF are shown in Box 2. The grades of recommendation for this position statement are listed below.12
A: Rich body of high-quality randomised controlled trial (RCT) data.
Routine instigation of non-pharmacological measures represents a central component of CHF management. This includes considering diet, exercise, and alcohol and fluid intake, as well as managing relevant comorbid conditions. These recommendations are summarised in Box 3.
Box 4 summarises pharmacological approaches to preventing and treating asymptomatic left-ventricular dysfunction. These include appropriate use of angiotensin-converting enzyme inhibitors (ACEIs) and β-blockers, as well as general management of patients with risk factors for ischaemic events.
Box 5 summarises recommendations for treatment of symptomatic chronic heart failure, divided into first-line agents (those that improve survival or alleviate symptoms), second-line agents, and others for patients who may have a specific need for such therapies.
Antiarrhythmic agents (apart from β-blockers and amiodarone) should be avoided because of their proarrhythmic potential, negative inotropic effects, and associated increased mortality.
Calcium antagonists that are direct negative inotropic agents, such as verapamil and diltiazem, are contraindicated in patients with systolic CHF. Dihydropyridine calcium antagonists such as amlodipine and felodipine do not improve survival in patients with systolic CHF, but can be used to treat comorbidities such as angina or hypertension in such patients.33,34,48
Tricyclic antidepressants and type-I antiarrhythmic agents should be avoided because of their proarrhythmic potential.
Non-steroidal anti-inflammatory drugs (NSAIDs) should be avoided, as they can inhibit the effects of diuretics and ACEIs, and can worsen both cardiac and renal function.49 Cyclooxygenase (COX)-2 inhibitors appear to have similar adverse effects on salt and water retention as do standard NSAIDs.50
Corticosteroids also have adverse effects on salt and water retention and should be avoided, if possible.
Thiazolidinediones, metformin and tumour necrosis factor antagonists should be used with caution in patients with CHF.
Positive inotropic agents can improve cardiac performance during short-term and long-term therapy. β-Adrenergic agonists (eg, dobutamine) and phosphodiesterase inhibitors (eg, milrinone) enhance cardiac contractility by increasing myocardial levels of cyclic adenosine monophosphate.51 However, despite favourable short-term haemodynamic and clinical effects, long-term oral therapy with positive inotropic agents has not been shown to reliably improve symptoms or clinical status, and has been associated with a significant increase in mortality.52-54 For similar reasons, long-term intermittent infusions of positive inotropic drugs are not recommended.
Levosimendan is a calcium-sensitising inotrope and vasodilator which may lead to improvements in outcomes compared with dobutamine and does not antagonise the effects of β-blockers.55 Further studies are ongoing to establish the place of this agent in the management of advanced systolic CHF.
Current recommendations for biventricular pacing and implantable cardioverter defibrillator (ICD) treatment of symptomatic CHF are summarised in Box 6. This is a rapidly developing and somewhat contentious area of heart failure management. Many patients who meet criteria for biventricular pacing will meet criteria for ICD, in which case they should receive devices that combine these therapies.
Left-ventricular aneurysmectomy may benefit patients with CHF in whom a large aneurysm can be excised, particularly if the remaining myocardium is functionally normal and there is minimal residual coronary artery disease.58
Left-ventricular free wall excision (frequently with concomitant mitral valve repair or replacement) aims to restore a normal myocardial mass-to-volume ratio in patients with severe left-ventricular dilatation. However, this procedure has not yet been subjected to clinical trials to define its place (if any) in managing CHF.59 Non-stimulated synthetic wrapping of the heart, which passively restricts left-ventricular dilatation, has been evaluated, with promising initial results.60
Left-ventricular assist devices (LVADs) are most often used as a temporary bridge to cardiac transplantation or for recovery of the heart after cardiac surgery.61 While they have occasionally been used as a long-term alternative to cardiac transplantation, no device has received regulatory approval for this indication. Their prohibitive cost and large size, the fact that only part of the device is implantable, and the risk of complications (especially infection and thromboembolism) limit the widespread use of currently available LVADs in patients with end-stage CHF.62 Surgical management of mitral regurgitation can produce significant improvement in both symptoms and left-ventricular function.63
Cardiac transplantation is an accepted therapy for certain patients with refractory CHF who meet eligibility criteria.64 The 5-year survival rate is 65%–75%, but a shortage of donors means it is available to very few patients.
Patients with coronary heart disease (CHD) who present with CHF (CHD–CHF) should be considered for coronary revascularisation after optimal pharmacological therapy has been started. Those with angina pectoris, a surrogate marker of viable ischaemic myocardium, are the more favourable candidates for coronary artery bypass graft surgery.65 However, patients with diabetes and CHD–CHF, who may not manifest angina as a symptom, warrant a more objective assessment of their myocardial status before excluding revascularisation as a therapeutic option.
For objective investigation of myocardial ischaemia and viability, dipyridamole and exercise thallium tests have been supplemented by positron emission tomography scanning and, more recently, cardiac magnetic resonance imaging. Individually, or in combination, these investigations can provide an objective assessment of the potential benefit of coronary revascularisation in most patients with CHD–CHF.66 However, there are no randomised controlled studies assessing the role of coronary revascularisation in treating heart failure symptoms.
The existence of heart failure with preserved systolic function (HFPSF) is universally accepted, although its precise definition, and hence, epidemiology, is subject to much debate. HFPSF is associated with an increased number of cardiovascular events and reduced survival, although less so than is systolic CHF.67 Recommendations for treatment are based on expert opinion only, as no randomised controlled trial has yet shown morbidity or mortality benefits in patients with HFPSF.68 Treatment comprises aggressive risk factor modification, including blood pressure reduction, strict glycaemic control and therapy directed against left-ventricular hypertrophy.69
CHF is often accompanied by important comorbid conditions that require specific intervention. These include concomitant ischaemic heart disease, valvular disease, arrhythmia, arthritis, gout, renal dysfunction, anaemia, diabetes, and sleep apnoea (Box 7).
Much of the burden of CHF is attributable to the large number of frail elderly patients in whom extensive comorbidity, poor coping skills, polypharmacy, and subsequent recurrent unplanned hospitalisations are common.76 To optimise the often complex management of such patients, a range of specialist management programs have been developed. The most successful of these programs have incorporated the following features:
targeting of high-risk (eg, frail elderly) individuals after acute hospitalisation;
multidisciplinary approach;
individualised care;
patient education and counselling (often involving the family or carer);
promotion of self-care behaviours;
intensive follow-up to detect and address clinical problems proactively;
strategies to apply evidence-based pharmacological treatment and to improve adherence;
application of non-pharmacological strategies where appropriate (eg, fluid and electrolyte management, and exercise programs); and
patient-initiated access to appropriate advice and support.76
Meta-analyses of randomised trials (including those undertaken in Australia) have shown that predominantly nurse-led, multi-disciplinary programs of care, delivered either in the home or through specialist CHF clinics, significantly reduce the risk of rehospitalisation, improve quality of life, reduce health care costs and prolong survival.77-81 Compared with treatment with ACEIs (numbers needed to treat [NNTs] to reduce mortality and CHF admissions being 19 and 16, respectively),82 these programs of care compare very favourably; the equivalent NNTs being 17 to reduce mortality and 10 to reduce admissions.78
Based on a favourable economic analysis of a United Kingdom-wide CHF service83 and the tangible benefits derived from the systematic management of CHF in New South Wales,84 CHF management programs are now proliferating widely throughout Australia.
Quality of life for patients with severe CHF refractory to optimal pharmacological and non-pharmacological strategies can be poor, and comparable to that of patients with terminal malignancies.85-87 Survival rates are as poor as for patients with the most common form of cancer, with a case-fatality rate of 75% over 5 years overall in those hospitalised with CHF.88
Palliative care should be considered for patients with the strong possibility of death within 12 months and who have advanced symptoms (ie, New York Heart Association Class IV) and poor quality of life, resistant to optimal pharmacological and non-pharmacological therapies.89-91 Strong markers of impending mortality include:
2 Recommendations for diagnostic investigation of chronic heart failure (CHF)
BNP = B-type natriuretic peptide. HFPSF = heart failure with preserved systolic function. |
|||||||||||||||
3 Recommendations for non-pharmacological management of chronic heart failure (CHF)
4 Recommendations for preventing chronic heart failure (CHF) and treating asymptomatic left-ventricular (LV) dysfunction
β-Blocker therapy should be commenced early after a myocardial infarction, whether or not the patient has systolic ventricular dysfunction.25,26 |
|||||||||||||||
5 Recommendations for pharmacological treatment of symptomatic chronic heart failure (CHF)
β-Blockers are also indicated for patients with symptoms of advanced CHF.41 |
|||||||||||||||
ACEI = angiotensin-converting enzyme inhibitor. LVEF = left-ventricular ejection fraction. |
|||||||||||||||
6 Recommendations for device-based treatment of symptomatic chronic heart failure (CHF)
- Henry Krum1
- Michael V Jelinek2,3
- Simon Stewart4,5
- Andrew Sindone6,7
- John J Atherton8,9
- Anna L Hawkes4
- on behalf of the CHF Guidelines Core Writers
- 1 NHMRC Centre of Clinical Research Excellence in Therapeutics, Department of Epidemiology and Preventive Medicine, and Department of Medicine, Monash University, Alfred Hospital, Melbourne, VIC.
- 2 University of Melbourne, Melbourne, VIC.
- 3 St Vincent's Hospital, Melbourne, VIC.
- 4 National Heart Foundation of Australia, Brisbane, QLD.
- 5 Baker Heart Research Institute, Melbourne, VIC.
- 6 University of Western Sydney, Sydney, NSW.
- 7 Concord Hospital, Sydney, NSW.
- 8 Royal Brisbane and Women's Hospital, Brisbane, QLD.
- 9 University of Queensland, Brisbane, QLD.
Most members of the core writing panel have received funding from or have associations with pharmaceutical companies. Details are available on The Medical Journal of Australia website at
- 1. McMurray JJV, Stewart S. The burden of heart failure. Eur Heart J 2003; 5: I3-I113.
- 2. Cowie MR, Struthers AD, Wood DA, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet 1997; 350: 1349-1353.
- 3. Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA 1996; 275: 1557-1562.
- 4. Australian Institute of Health and Welfare and the National Heart Foundation of Australia. Heart, stroke and vascular diseases — Australian facts 2004. Canberra: National Centre for Monitoring Cardiovascular Disease, 2004: 140.
- 5. Abhayaratna WP, Smith WT, Becker NG, et al. Prevalence of heart failure and systolic ventricular dysfunction in older Australians: the Canberra Heart Study. Med J Aust 2006; 184: 151-154. <MJA full text>
- 6. Clark RA, McLennan S, Dawson A, et al. Uncovering a hidden epidemic: a study of the current burden of heart failure in Australia. Heart Lung Circ 2004; 13: 266-273.
- 7. Krum H, Tonkin AM, Currie R, et al. Chronic heart failure in Australian general practice: the Cardiac Awareness Survey And Evaluation (CASE) study. Med J Aust 2001; 174: 439-444. <MJA full text>
- 8. Access Economics. The shifting burden of cardiovascular disease in Australia. Canberra: Access Economics Pty Ltd and the National Heart Foundation of Australia, May 2005.
- 9. Berger R, Huelsman M, Strecker K, et al. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 2002; 105: 2392-2397.
- 10. Stevenson LW, Tillisch JH, Hamilton M, et al. Importance of hemodynamic response to therapy in predicting survival with ejection fraction less than or equal to 20% secondary to ischemic or non-ischemic dilated cardiomyopathy. Am J Cardiol 1990; 66: 1348-1354.
- 11. McCarthy RE, Boehmer JP, Hruban RH, et al. Long-term outcome of fulminant myocarditis as compared with acute (non-fulminant) myocarditis. N Engl J Med 2000; 342: 690-695.
- 12. National Health and Medical Research Council. Clinical practice guidelines for the management of overweight and obesity in children and adolescents. Canberra: NHMRC, 2003.
- 13. Mancini DM, Walter G, Reichek N, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 1992; 85: 1364-1373.
- 14. Chati Z, Zannad F, Jeandel C, et al. Physical deconditioning may be a mechanism for the skeletal muscle energy phosphate muscle metabolism abnormalities in chronic heart failure. Am Heart J 1996; 131: 560-566.
- 15. Meyer K, Schwaibold M, Westbrook S, et al. Effects of exercise training and activity restriction in 6-minute walking test performance in patients with chronic heart failure. Am Heart J 1997; 133: 447-453.
- 16. Sinoway LI. Effect of conditioning and deconditioning stimuli on metabolically determined blood flow in humans and implications for congestive heart failure. Am J Cardiol 1988; 62: 45E-48E.
- 17. Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995; 333: 1190-1195.
- 18. Stewart S, Vandenbroek AJ, Pearson S, Horowitz JD. Prolonged beneficial effects of a home-based intervention on unplanned readmissions and mortality among patients with congestive heart failure. Arch Intern Med 1999; 159: 257-261.
- 19. Naughton MT. Impact of treatment of sleep apnoea on left ventricular function in congestive heart failure. Thorax 1998; 53: S37-S40.
- 20. McDonald CD, Burch GE, Walsh JJ. Prolonged bed rest in the treatment of idiopathic cardiomyopathy. Am J Med 1972; 52: 41-50.
- 21. Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30: 725-735.
- 22. Zusman RM, Morales A, Glasser DB, Osterloh IH. Overall cardiovascular profile of sildenafil citrate. Am J Cardiol 1999; 83: 35C-44C.
- 23. Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the Survival and Ventricular Enlargement Trial. N Engl J Med 1992; 327: 669-677.
- 24. The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992; 327: 685-691.
- 25. Medical Research Council Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ 1992; 304: 405-412.
- 26. Dahlof B, Lindholm JH, Hansson L, et al. Morbidity and mortality in the Swedish trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338: 1281-1285.
- 27. Kostis JB, Davis BR, Cutler J, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research group. JAMA 1997; 278: 212-216.
- 28. Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet 1999; 353: 611-616.
- 29. Brown MJ, Palmer CR, Castaigne A, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS Study: intervention as a goal in hypertension treatment (INSIGHT). Lancet 2000; 356: 366-372.
- 30. Hansson L, Hedner T, Lund-Johansen P, et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000; 356: 359-365.
- 31. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000; 342: 145-153.
- 32. Kjekshus J, Pederson TR, Olsson AG, et al. The effects of simvastatin on the incidence of heart failure in patients with coronary heart disease. J Card Fail 1997; 2: 249-254.
- 33. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996; 334: 1349-1355.
- 34. Cohn JN, Ziesche S, Smith R, et al. Effect of the calcium antagonist, felodipine as supplementary vasodilator therapy in patients with chronic heart failure treated with enalapril: V-HeFT III. Vasodilator-Heart Failure Trial (V-HeFT) Study Group. Circulation 1997; 96: 856-863.
- 35. The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fraction and congestive heart failure. N Engl J Med 1991; 325: 293-302.
- 36. The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316: 1429-1435.
- 37. The NETWORK Investigators. Clinical outcome with enalapril in symptomatic chronic heart failure: a dose comparison. Eur Heart J 1998; 19: 481-489.
- 38. Packer M, Poole-Wilson PA, Armstrong PW, et al. Comparative effects of low and high doses of angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation 1999; 100: 2312-2318.
- 39. CIBIS II Investigators and Committees. The cardiac insufficiency bisoprolol study II (CIBIS II): a randomised trial. Lancet 1999; 353: 9-13.
- 40. Merit-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353: 2001-2007.
- 41. Packer M, Coates AJ, Fowler MB, et al, for the Carvedilol Prospective Randomised Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the survival of patients with severe chronic heart failure. N Engl J Med 2001; 344: 1651-1658.
- 42. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341: 709-717.
- 43. Pitt B, Segal R, Martinez FA, et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet 1997; 349: 747-752.
- 44. McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362: 767-771.
- 45. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336: 525-533.
- 46. Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative study. N Engl J Med 1986; 314: 1547-1552.
- 47. Packer M, O’Connor CM, Ghali JK, et al. Effect of amlodipine on survival. Evaluation Study Group. N Engl J Med 1996; 335: 1107-1114.
- 48. Thackray S, Witte K, Clark AL, Cleland JG. Clinical trials update: OPTIME-CHF, PRAISE-2, ALL-HAT. Eur J Heart Fail 2000; 2: 209-212.
- 49. Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an under-recognized public health problem. Arch Intern Med 2000; 160: 777-784.
- 50. Swan SK, Rudy DW, Lasseter KC, et al. Effect of cyclooxygenase-2 inhibition on renal function in elderly persons receiving a low-salt diet. A randomized, controlled trial. Ann Intern Med 2000; 133: 1-9.
- 51. Keteyian SJ, Brawner CA, Schairer JR, et al. Exercise training in patients with heart failure: a randomised, controlled trial. Ann Intern Med 1996; 124: 1051-1057.
- 52. Packer M, Carver JR, Rodeheffer RJ, et al, for the PROMISE Study Reseach Group. Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med 1991; 325: 1468-1475.
- 53. Hampton JR, Van Veldhuisen DJ, Kleber FX, et al, for the Second Prospective Randomised Study of Ibopamine on Mortality and Efficacy (PRIME II) Investigators. Randomised study of effect of ibopamine on survival in patients with advanced severe heart failure. Lancet 1997; 349: 971-977.
- 54. The Xamoterol in Severe Heart Failure Study Group. Xamoterol in severe heart failure. Lancet 1990; 336: 1-6.
- 55. Follath F, Cleland JG, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (LIDO study): a randomised double-blind trial. Lancet 2002; 360: 196-202.
- 56. Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001; 344: 873-880.
- 57. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350: 2140-2150.
- 58. Dor V, Sabatier M, Di Donato M, et al. Efficacy of endoventricular patch plasty in large postinfarction akinetic scar and severe left ventricular dysfunction: comparison with a series of large dyskinetic scars. J Thorac Cardiovasc Surg 1998; 116: 50-59.
- 59. Dreyfus G, Mihealainu S. The Batista procedure. Heart 2001; 85: 1-2.
- 60. Raman JS, Power JM, Buxton B, et al. Ventricular containment as an adjunctive procedure in ischaemic cardiomyopathy: early results. Ann Thorac Surg 2000; 70: 1124-1126.
- 61. Jaski BE, Lingle RJ, Reardon LC, Dembitsky WP. Left ventricular assist device as a bridge to patient and myocardial recovery. Prog Cardiovasc Dis 2000; 43: 5-18.
- 62. Rose EA, Gelijns C, Moskowitz AJ, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med 2001; 345: 1435-1443.
- 63. Bolling SF, Pagani FD, Deeb GM, Bach DS. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg 1998; 115: 381-388.
- 64. Dabol R, Edwards NM. Cardiac transplantation and other therapeutic options in the treatment of end-stage heart disease. Compr Ther 2000; 26: 109-113.
- 65. Pagano D, Bonser RS, Camici PG. Myocardial revascularization for the treatment of post-ischemic heart failure. Curr Opin Cardiol 1999; 14: 506-509.
- 66. Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol 2002; 39: 1151-1158.
- 67. Redfield MM, Jacobsen SJ, Burnett JC Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003; 289: 194-202.
- 68. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003; 362: 777-781.
- 69. Schmieder RE, Martus P, Klingbeil A. Reversal of left ventricular hypertrophy in essential hypertension: a meta-analysis of randomized double-blind studies. JAMA 1996; 275: 1507-1513.
- 70. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347: 1825-1833.
- 71. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352: 225-237.
- 72. Frances CD, Noguchi H, Massie BM, et al. Are we inhibited? Renal insufficiency should not preclude the use of ACE inhibitors for patients with myocardial infarction and depressed left ventricular function. Arch Intern Med 2000; 160: 2645-2650.
- 73. Silverberg DS, Wexler D, Blum M, et al. The effect of correction of anaemia in diabetics and non-diabetics with severe resistant congestive heart failure and chronic renal failure by subcutaneous erythropoietin and intravenous iron. Nephrol Dial Transplant 2003; 18: 141-146.
- 74. Haas SJ, Vos T, Gilbert RE, Krum H. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trials. Am Heart J 2003; 146: 848-853.
- 75. Massie BM, Krol WF, Ammon SE, et al. The Warfarin and Antiplatelet Therapy in Heart Failure trial (WATCH): rationale, design, and baseline patient characteristics. J Card Fail 2004; 10: 101-112.
- 76. Rich MW. Heart failure disease management: a critical review. J Card Fail 1999; 5: 64-75.
- 77. McAlister FA, Lawson FM, Teo KK, Armstrong PW. A systematic review of randomized trials of disease management programs in heart failure. Am J Med 2001; 110: 378-384.
- 78. McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004; 44: 810-819.
- 79. Phillips CO, Wright SM, Ker DE, et al. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. JAMA 2004; 291: 1358-1367.
- 80. Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomized controlled study. Lancet 1999; 354: 1077-1083.
- 81. Stewart S, Horowitz JD. Home-based intervention in congestive heart failure: long-term implications on readmission and survival. Circulation 2002; 105: 2861-2866.
- 82. Flather MD, Yusuf S, Kober L, et al. Long-term ACE inhibitor therapy in patients with heart failure or left ventricular dysfunction: a systematic overview of data from individual patients. Lancet 2000; 355: 1575-1581.
- 83. Stewart S, Blue LE. Specialist nurse intervention in chronic heart failure: from research to practice. London: BMJ Books, 2004.
- 84. NSW Department of Health. Chronic care program 2000-2003: strengthening capacity for chronic care in the NSW health system. Clinical Services Framework for heart failure. Overview of the framework and its standards. Volume 1. Sydney: NSW Health, 2003.
- 85. Hinton JM. The physical and mental distress of dying. Q J Med 1963; 32: 1-21.
- 86. Gibbs LM, Addington-Hall J, Gibbs SJ. Dying from heart failure: lessons from palliative care. BMJ 1998; 317: 961-962.
- 87. Stewart A, Greenfield S, Hays RD, et al. Functional status and well being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA 1989; 262: 907-913.
- 88. Stewart S, MacIntyre K, Hole DJ, et al. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Failure 2001; 3: 315-322.
- 89. Goodlin SJ, Jette AM, Lynn J, Wasson JH. Community physicians describe management issues for patients expected to live less than twelve months. J Palliat Care 1998; 14: 30-35.
- 90. Alla F, Briancon S, Guillemin F, et al. Self-rating of quality of life provides additional prognostic information in heart failure. Insights into the EPICAL study. Eur J Heart Fail 2002; 4: 337-343.
- 91. Stewart S, McMurray JJ. Palliative care for heart failure. BMJ 2002; 325: 915-916.





Abstract
Chronic heart failure (CHF) is found in 1.5%–2.0% of Australians. Considered rare in people aged less than 45 years, its prevalence increases to over 10% in people aged ≥ 65 years.
CHF is one of the most common reasons for hospital admission and general practitioner consultation in the elderly (≥ 70 years).
Common causes of CHF are ischaemic heart disease (present in > 50% of new cases), hypertension (about two-thirds of cases) and idiopathic dilated cardiomyopathy (around 5%–10% of cases).
Diagnosis is based on clinical features, chest x-ray and objective measurement of ventricular function (eg, echocardiography). Plasma levels of B-type natriuretic peptide (BNP) may have a role in diagnosis, primarily as a test for exclusion. Diagnosis may be strengthened by a beneficial clinical response to treatment(s) directed towards amelioration of symptoms.
Management involves prevention, early detection, amelioration of disease progression, relief of symptoms, minimisation of exacerbations, and prolongation of survival.