Studies examining the link between research evidence and clinical practice have consistently shown gaps between the evidence and current practice. Some studies in the United States suggest that 30%–40% of patients do not receive evidence-based care, while in 20% of patients care may be not needed or potentially harmful.1 However, relatively little information exists about how to apply evidence in clinical practice, and data on the effect of evidence-based guidelines on knowledge uptake, process of care or patient outcomes is limited.
In Australia and New Zealand, national guidelines for treating patients with chronic kidney disease — the Caring for Australasians with renal impairment (CARI) guidelines2 — were published and disseminated to nephrologists in March 2000, with details and updates on the CARI website (http://www.cari.org.au). They provide nephrologists, renal nurses and other health carers with an evidence base for patient management and improving outcomes.
The focus of one of the CARI guidelines2 is anaemia, a common complication of chronic kidney disease. Management of iron levels in patients with chronic kidney disease involves both excluding iron deficiency in uraemic–anaemic patients, and providing adequate iron stores to allow patients to efficiently maintain target haemoglobin concentrations, especially with the concomitant use of supplementary erythropoietin proteins (epoetin). Failure to achieve adequate iron stores and availability is the major cause of epoetin resistance, which may result in increased costs to correct the anaemia.3 In observational studies of haemodialysis patients, it has been shown that the relative risk of death and hospitalisation increases significantly with haemoglobin levels below the target.4
In an effort to understand the impact of guidelines, our study was designed to evaluate the outcomes of a standard implementation strategy (passive dissemination of guidelines in hardcopy form and on the Internet) of the CARI guidelines using an example — iron management of dialysis patients in Australia. In assessing this strategy, we sought to identify barriers to guideline implementation5-7 using a “process of care” approach, with a view to developing strategies to increase uptake of evidence into practice. We hope that lessons learned from this process can be applied in other clinical environments.
From the CARI guidelines biochemical and haematological targets,2 the guideline for iron was chosen for this study because:
it has high levels of supporting evidence;
it is of clinical relevance to all renal units;
there are potentially high costs associated with not applying this guideline (related to greater epoetin product use); and
it involves easily measured parameters, and the necessary data are collected by the Australian and New Zealand Dialysis and Transplant Registry (ANZDATA; <http://www.anzdata.org.au>).8
The evolution of the evidence base has led to changes in target levels. Initially, the CARI guidelines (March 2000) set a target minimum haemoglobin level of 120 g/L for patients having dialysis for chronic kidney disease. The current revised minimum haemoglobin target level is 110 g/L, but, as previously, the level should not exceed 120 g/L for patients with diabetes or established additional cardiovascular risk.2 For patients having dialysis, the target ranges of iron values are: serum ferritin level, 300– 800 μg/L; and transferrin saturation (TSAT), 20%–50%, and/or percentage of hypochromic red blood cells < 2.5%.
ANZDATA records incidence, prevalence and outcome data for all patients treated for end-stage kidney disease. Relevant to the current study, ANZDATA also routinely collects demographic, haematological and biochemical data, type of dialysis, dialysis prescription, and complications and death rates.8 For our review, with specific permission from the Units concerned, de-identified data on patients’ haemoglobin levels and the results of iron studies (serum ferritin and TSAT), as well as demographic characteristics, were released by ANZDATA. These data were from the March 2004 ANZDATA survey, and the iron studies were the most recent for each patient before that date.
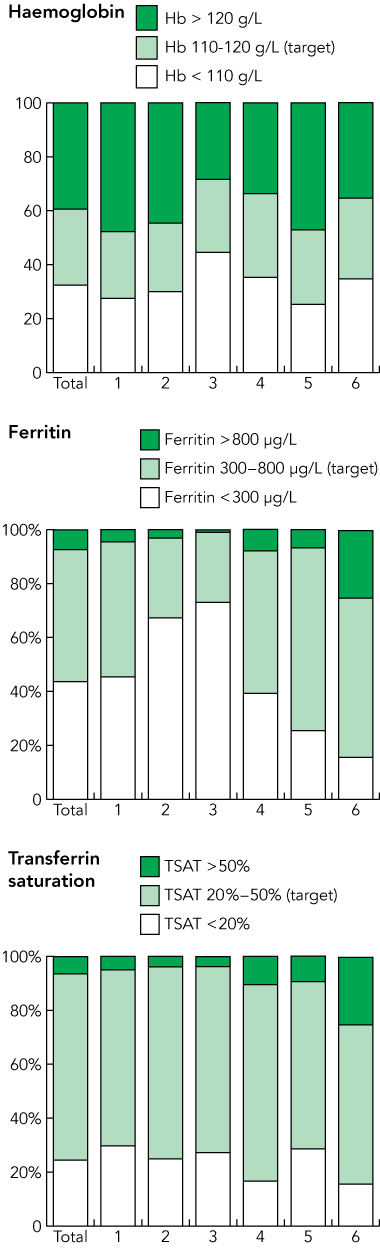
There were 1763 patients in the dataset from the six units (Box 1). Statistically significant differences were found between median values for haemoglobin (P < 0.001), ferritin (P < 0.001) and TSAT levels (P < 0.001). Among the units, patients’ median haemoglobin levels ranged from 112 g/L to 121 g/L, median ferritin levels from 163 μg/L to 501 μg/L, and median TSAT percentages varied from 23% to 29%.
Box 2 shows the proportion of patients who were within or outside CARI target iron parameters for each unit. The proportions were significantly different across the units. The greatest difference was in ferritin levels in Unit 3 compared with Unit 5, with 26% versus 68% of patients in the target range, respectively.
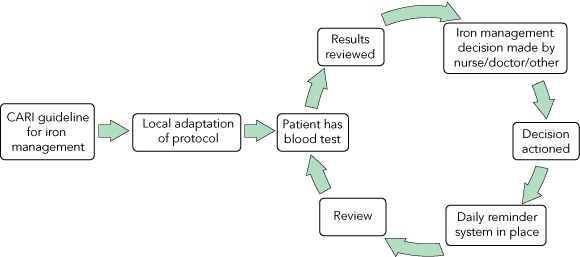
Box 3 shows the process pathway for iron management across the units. Each unit varied each of the steps depending on local protocols. Practices differed widely from the CARI guideline and between the units. Most units agreed with the CARI guideline on the lower margin of the range for iron stores, but there was a tendency for all units in their local adaptation of the guideline to adopt a lower level for the upper limit for iron stores. Units also varied widely in the frequency with which iron studies were undertaken, as well as whether oral or intravenous iron therapy was administered and what dosages were used.
The process for iron management was different for each unit. All units had a written iron protocol, but not all units complied with their protocol. Units 1, 5 and 6 had a written, agreed and implemented protocol. Units 1 and 5 had a standing order for iron that allowed nursing staff to administer iron within this specified protocol. Units 1, 5 and 6 had a decision aid for administering iron. Many variables affected the iron management process. A summary of each unit’s iron management process is given in Box 4.
The possible barriers to more successful implementation of the guideline, which were identified from the results of the review of the six units, are listed in Box 5.
Every step in the iron management clinical process pathway (Box 3) contains factors influencing iron management. Identifying strengths and weaknesses in this process for individual units will aid implementation. As our study is primarily a qualitative study, drawing statistical inferences is difficult. However, there appears to be a link between achieving higher ferritin concentrations and autonomy given to nursing staff to manage patients’ iron levels under an agreed protocol. Other factors that appear to influence guideline adherence and patient outcomes are:
agreement between nursing staff and nephrologists on a protocol for the unit;
using an effective decision aid (Box 6);
the number of nephrologists practising in a particular unit (negative effect with increasing numbers);
the degree of physician reliance on a protocol being actively implemented; and
the iron management protocol being “proactive” rather than “reactive”. Evidence suggests that a proactive or maintenance-dosing iron therapy regimen is superior to a reactive regimen (ie, only prescribing iron therapy when iron indices are outside the defined ranges).3,9,10
Some dialysis facilities had lower target haemoglobin concentrations and lower achieved levels, possibly due to concerns about increased thrombotic risk and mortality rates. These concerns were raised by the publication of a randomised controlled trial linking an increase in mortality to high haemoglobin concentrations in haemodialysis patients with symptomatic heart failure.11 Another barrier to aggressive iron administration is a reluctance on the part of nephrologists to allow ferritin levels to become “too high”. Although all agreed that the lower limit for ferritin should be > 300 μg/L, some believed that the CARI guideline upper limit of 800 μg/L was too high and exposed patients to the risk of iron toxicity (ie, increased risk of infection, oxidative stress,12 and impaired neutrophil function).13 Some dialysis units subsequently adopted a revised local protocol, lowering target ferritin concentrations to differing extents. This change in practice is reflected in the new evidence-based CARI guideline published in April 2006 in which the upper limit for ferritin has been reduced to 500 μg/L.14
The sample had a lower proportion of Aboriginal and Torres Strait Islander patients compared with the overall Australian dialysis population. Indigenous patients have been reported to have lower ferritin and TSAT values than non-Indigenous patients, and may require different iron management processes.15 Further research into the needs of Indigenous patients having dialysis is required to determine their particular requirements and the applicability of the CARI guideline to Indigenous patients.
There is a growing body of research on how evidence is taken up into clinical practice. The most common strategies in use — continuing medical education and passive dissemination of guidelines — have consistently been shown to have very little impact on practice patterns or improving patient outcomes.7,16-19 For successful implementation of guidelines, it is necessary to devise a strategy or plan for the project.6-8 The first task is to understand the local setting for implementation and the target group,20 as well as the current process or clinical pathway that needs to be altered (Box 3). Understanding each step in the clinical pathway and how individual units move through these stages will reveal the barriers to change for those units,5-7,20 and a multifaceted implementation plan can be devised to overcome these barriers.16,19-21
In our study, identification of barriers was made at seven different levels of the organisation, using the National Institute for Clinical Studies barrier tool.22 Box 5 shows the many possible barriers at all units, involving nephrologists, renal nurses, patients, and issues at a unit level, management level or infrastructure level, as well as the guideline itself. Individual units wanting to implement the iron guideline can identify which barriers may be applicable to them and devise strategies to overcome these. Once these barriers have been overcome, regular audits are needed to ensure that the performance indicators have improved (ie, the proportion of patients achieving targets has increased). This completes the quality cycle, ensuring ongoing delivery of optimal evidence-based care.
1 Demographic characteristics, iron scores and dialysis details of patients at six renal units
IQR = interquartile range; TSAT = transferrin saturation. * χ2 test or Kruskal–Wallis test used for univariate analysis among units. |
|||||||||||||||
2 Haemoglobin and iron indices ranges (all units) by dialysis unit (1 to 6) for patients taking epoetin
4 Summary of each dialysis unit’s iron management processes
5 Possible barriers to successful implementation of the iron guideline
Lack of awareness or knowledge of a guideline
Lack of knowledge regarding iron requirements
Lack of “trust” in the guideline
Lack of ability to implement the guideline in own practice
Lack of awareness or knowledge of guideline
Lack of knowledge regarding iron requirements
Has to follow/wait for instruction from nephrologist regarding iron management
Possible increased workload
Following up home dialysis patients
Not accepting iron as important
Side effects from prescribed treatments
Comorbid conditions may take precedence
May be a home dialysis patient
Large numbers of nephrologists working within the one dialysis unit
Lack of agreement on iron targets among nephrologists
Lack of effective iron protocol available for staff to follow
Lack of care plan available for staff to follow for iron management
May not realise that iron management is an issue
Unaware of the reduction in relative cost of anaemia management with epoetin, by provision of adequate iron
Increased nursing time to check laboratory results of iron studies
Lack of computerised results
Laboratories do not automatically send blood test results to dialysis units; nurses are required to access results for their patients
Iron measurements come from a range of laboratories with different ordering processes and accessibility of results
Received 10 October 2005, accepted 23 March 2006
- Michelle J Irving1
- Jonathan C Craig1,2
- Martin Gallagher3
- Stephen McDonald4
- Kevan R Polkinghorne5
- Rowan G Walker6
- Simon D Roger7
- 1 Centre for Kidney Research, The Children's Hospital at Westmead, Sydney, NSW.
- 2 School of Public Health, University of Sydney, Sydney, NSW.
- 3 Renal Unit, The Canberra Hospital, Canberra, ACT.
- 4 Australian and New Zealand Dialysis and Transplant Registry (ANZDATA), Adelaide, SA.
- 5 Nephrology Department, Monash Medical Centre, Melbourne, VIC.
- 6 Renal Unit, Royal Melbourne Hospital, Melbourne, VIC.
- 7 Renal Unit, Gosford Hospital, Gosford, NSW.
Simon Roger has made presentations to Sigma Pharmaceuticals (iron polymaltose), and is a past chairman of the now disbanded advisory board of Baxter Healthcare (iron polymaltose). He has received funding from Vifor (iron polymaltose) for an investigator-initiated trial into oral versus intravenous iron polymaltose in patients with chronic kidney disease.
Stephen McDonald has received travel assistance from Amgen Australia, and until March 2005 his salary was partly funded by a grant from Amgen Australia to the ANZDATA Registry.
These organisations had no role in the analysis and preparation of the article.
The ANZDATA Registry has received donations from Amgen Australia and Janssen-Cilag (products for treating renal anaemia).
- 1. Schuster MA, McGlynn EA, Brook RH. How good is the quality of health care in the United States? Milbank Q 1998; 76: 517-563.
- 2. Council of the Australian and New Zealand Society of Nephrology (ANZSN) and Kidney Health Australia (KHA). Caring for Australasians with renal impairment (CARI) guidelines. 2004. http://www.cari.org.au/index.php (accessed Mar 2006).
- 3. Besarab A, Amin N, Ahsan M, et al. Optimization of epoetin therapy with intravenous iron therapy in hemodialysis patients. J Am Soc Nephrol 2000; 11: 530-538.
- 4. Locatelli F, Pisoni RL, Akizawa T, et al. Anemia management for hemodialysis patients: Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines and Dialysis Outcomes and Practice Patterns Study (DOPPS) findings. Am J Kidney Dis 2004; 44 (5 Suppl 2): 27-33.
- 5. Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet 2003; 362: 1225-1230.
- 6. Gros PA, Greenfield S, Cretin S, et al. Optimal methods for guideline implementation: conclusions from Leeds Castle meeting. Med Care 2001; 39 (8 Suppl 2): II85-II92.
- 7. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care 2001; 39 (8 Suppl 2): II46-II54.
- 8. Australia and New Zealand Dialysis and Transplant Registry. Adelaide: ANZDATA, 1998-2001. http://www.anzdata.org.au (accessed Jul 2006).
- 9. Richardson D, Bartlett C, Will EJ. Optimizing erythropoietin therapy in hemodialysis patients. Am J Kidney Dis 2001; 38: 109-117.
- 10. Besarab A, Kaiser JW, Frinak S. A study of parenteral iron regimens in hemodialysis patients. Am J Kidney Dis 1999; 34: 21-28.
- 11. Besarab A, Bolton WK, Browne J, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339: 584-590.
- 12. Roob JM, Khoschsorur G, Tiran A, et al. Vitamin E attenuates oxidative stress induced by intravenous iron in patients on hemodialysis. J Am Soc Nephrol 2000; 11: 539-549.
- 13. Waterlot Y, Cantinieaux B, Hariga-Muller C, et al. Impaired phagocytic activity of neutrophils in patients receiving hemodialysis: the critical role of iron overload. BMJ 1985; 291: 501-504.
- 14. Roger S. Haematological targets: iron. Nephrology (Carlton) 2006; 11 Suppl 1: S217-S219.
- 15. Snelling P, Irish A. Do Aboriginal and Torres Strait Islander patients meet biochemical and haematological targets from the CARI guidelines [abstract]. Nephrology (Carlton) 2004; 9 (Suppl): A25.
- 16. NHS Centre for Reviews and Dissemination: effective health care — getting evidence into practice. The University of York Bulletin 1999; 5 (1): 1-16.
- 17. Bero LA, Grilli R, Grimshaw JM, et al. Closing the gap between research and practice: an overview of systemic reviews of intervention to promote the implementation of research findings. BMJ 1998; 317: 465-468.
- 18. Eccles MP, Grimshaw J. Selecting, presenting and delivering clinical guidelines: are there any “magic bullets”? Med J Aust 2004; 180: S52-S54. <MJA full text>
- 19. Grimshaw JM, Shirran LS, Thomas R, et al. Changing provider behaviour: an overview of systemic reviews of interventions. Med Care 2001; 39 (8 Suppl 2): II2-II45.
- 20. Grol R, Wensing M. What drives change? Barriers to and incentives for achieving evidence-based practice. Med J Aust 2004; 180: S57-S60.
- 21. Locatelli F, Andrulli S, Del Vecchio L. Difficulties of implementing clinical guidelines in medical practice. Nephrol Dial Transplant 2000; 15: 1284-1287.
- 22. National Institute of Clinical Studies (NICS). The NICS barrier tool. Melbourne: NICS, 2005. http://www.nicsl.com.au/asp/index.asp?page=knowledge/knowledge_article_type&cid=5212&id=417 (accessed Sept 2006).





Abstract
Objective: To evaluate the outcomes of and barriers to implementing standard guidelines (Caring for Australasians with renal impairment [CARI]), using iron management in patients having dialysis as an example.
Design and setting: On-site review of iron management processes at six Australian dialysis units varying in size and locality. Patients’ iron indices and haemoglobin levels were obtained from the Australian and New Zealand Dialysis and Transplant Registry.
Participants: Patients with chronic kidney disease who were dependent on dialysis.
Main outcome measures: Processes for assessing indices of iron stores and iron supplementation; comparison with target indices in the CARI guidelines.
Results: There was considerable variability among the units in achievement of haemoglobin and iron targets, with 25%–32% of patients achieving haemoglobin targets of 110–120 g/L, 30%–68% achieving ferritin targets of 300–800 μg/L, and 65%–73% achieving transferrin saturation targets of 20%–50%. Implementation barriers included lack of knowledge, lack of awareness of or trust in the CARI guideline, inability to implement the guideline, and inability to agree on a uniform unit protocol. Factors associated with achieving the CARI guideline targets included nurse-driven iron management protocols, use of an iron management decision aid, fewer nephrologists per dialysis unit, and a “proactive” (actively keeping iron levels within target range) rather than “reactive” (only reacting if iron levels are out of the range) protocol.
Conclusions: Variability in achievement of iron targets, despite the availability of a clinical practice guideline, may be explained by variability in processes of care for achieving and maintaining adequate iron parameters.