Available, published, cross-sectional data show that mortality from prostate cancer is currently higher in rural than urban areas of Australia,1 but this might not have always been the case. Specifically, it has been reported that mortality rates for prostate cancer were similar in urban and rural areas of New South Wales until 1988, when a rural excess first became apparent.2 To clarify whether there has been a difference in urban–rural mortality trends, we examined official Australian data on prostate cancer mortality for 1985 to 2002.
We also investigated what relationship, if any, exists between urban–rural differences in prostate cancer mortality, radical prostatectomy and prostate-specific antigen (PSA) testing. In a climate of continuing uncertainty about the benefits of detecting and treating asymptomatic prostate cancer,3 it would not be unusual to find geographical variations in radical prostatectomy and PSA testing. For example, a recent Australian workshop on informed choice for prostate cancer screening heard anecdotal evidence that the rate of PSA screening among rural men might be lower than for their urban counterparts.4
The Australian Bureau of Statistics (ABS) uses the Australian Standard Geographic Classification (ASGC)5 to collect and disseminate official statistics by geographical area. For this study, we used Statistical Divisions (SDs), a component of the ASGC, to define capital city areas. In the ASGC, capital city SDs are designed to contain the anticipated development of a city for a period of at least 20 years and provide a geographical definition that is stable over time for analytical purposes.
Because there is a low incidence of prostate cancer among men younger than 50 years,6 and a perceived difficulty in establishing the cause of death among the oldest of the elderly,7 we limited our analysis to men aged 50–79 years. Sixty-one per cent of Australian men aged 50–79 years live in capital city SDs; we compared this group with men aged 50–79 years living in regional and rural areas of Australia. Our analyses of mortality, incidence, PSA testing and radical prostatectomy were based on place of usual residence, which might not necessarily be the same as the place of death, diagnosis, testing or treatment.
We obtained data on death registrations and populations from the ABS, data on prostate cancer incidence and radical prostatectomy from the Australian Institute of Health and Welfare, and data on PSA testing from the Medicare Benefits Schedule (MBS). Box 1 shows the codes and the time periods used; we included the earliest year for which there were consistent data stratified by SD. All data were provided in aggregated, non-identifiable, anonymous format from routinely maintained collections and were based on large geographical areas (ie, capital city SDs versus the rest). These highly aggregated data are normally used for routine public health surveillance, so we did not seek ethical approval.
In this article, we use the term “PSA testing” rather than “PSA screening” to refer to the MBS data, as there is no information about whether the test was used to screen asymptomatic men or to monitor disease. A previous report compared MBS data with self-reported screening data and concluded that most PSA testing currently done in Australia is for screening.8
We calculated directly age-standardised rates for each of the outcome measures using the 2001 Australian population as the standard. Trends in age-standardised rates were calculated using joinpoint regression, which estimates changes in the size or direction of the linear trend over time.9
Point estimates and confidence intervals for the ratio of the directly age-standardised rates were obtained from the statistical package Stata,10 which uses a previously described method.11 Trends in rate ratios for mortality over time were assessed by means of Poisson regression models.
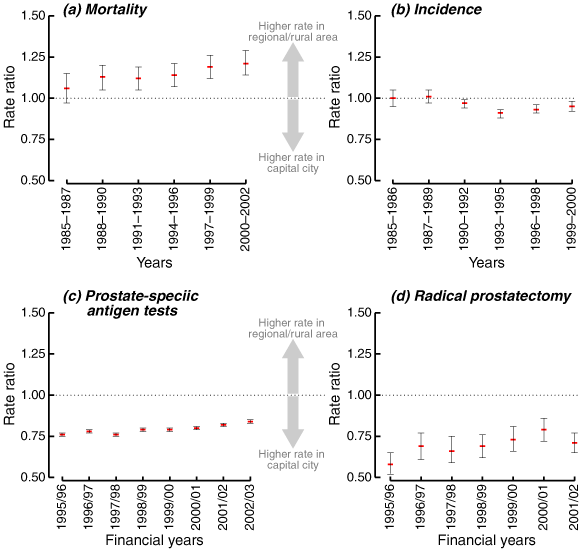
Our findings are summarised as age-standardised rates (Box 2) and as trends in the differentials between captal city residents and regional and rural residents (Box 3) for our four outcome measures: mortality and incidence of prostate cancer, PSA tests and radical prostatectomy.
Prostate cancer mortality started to decrease in 1993 among men who lived in both capital cities and regional and rural areas (Box 2[a]). In the 3 years 1985–1987, the prostate cancer mortality rate for regional and rural men was 6% higher than for capital city men, but this was not statistically significant (95% CI, 3% lower to 15% higher — see Box 3[a]). However, this difference increased over time, so that for the final 3-year period in this study (2000–2002) it had reached 21% (95% CI, 14% higher to 29% higher). The increase in rate ratios was statistically significant (annual increase, 2.3%; 95% CI, 1.1%–3.5%). As suggested by these results, the decrease in mortality since 1993 was larger for capital cities (annual change, −3.5%) than regional and rural areas (− 2.8%). By applying the age-specific rates in capital cities to the regional and rural population for 1998–2002, we found that regional and rural areas had a total of 110 extra deaths from prostate cancer per year than capital cities.
There was a dramatic increase in the incidence of prostate cancer in both capital city and regional and rural areas between 1990 and 1994 (Box 2[b]) — this was related to the surge in PSA testing.10 Although the number of PSA tests performed continued to increase after 1994 (see below), the incidence of prostate cancer decreased, probably because prevalent cases had been identified and removed from the group of men being screened.12 Box 3(b) shows that we found no statistically significant differences in incidence between capital cities and regional and rural areas until 1994, when men living in regional and rural Australia had rates that were 9% lower (95% CI, 12% lower to 7% lower) than those living in capital cities. By 1999–2000, this had decreased to 5% lower (95% CI, 8% lower to 2% lower).
Geographical data are only available for PSA testing and radical prostatectomy from the financial year 1995/96. Men living in regional and rural areas were less likely to have PSA tests than men living in capital cities, but this difference decreased over time (Box 2[c], Box 3[c]). The pattern was similar for radical prostatectomy. In 2001/02, men living in regional and rural areas had rates of radical prostatectomy that were 29% lower (95% CI, 35% lower to 23% lower) than their capital city counterparts. In 1995/96 the difference was 42% (95% CI, 48% lower to 35% lower — see Box 2[d], Box 3[d]).
Our analyses confirmed previously published work which found that prostate cancer mortality in Australia started to decrease in about 1993.13 It also provides three important pieces of new information:
The excess of prostate cancer mortality in regional/rural areas is increasing (for 2000–2002 the excess was 21%);
Rates of radical prostatectomy in regional/rural Australia are 29% lower than in the capital cities; and
Although PSA testing is common across the whole of Australia (about a quarter of men aged 50–79 years are tested annually8) it is less common in regional/rural areas than in the capital cities.
It is unlikely that PSA testing is obscuring higher rates of particularly aggressive prostate cancer in regional and rural Australia, as incidence was similar in urban and regional/rural areas before 1990 and the widespread use of PSA testing. Therefore, the most likely reasons for the difference involve differences in management. This should not be surprising, because previous studies have found urban–rural differences in the management of other cancers. For example, women with breast cancer in rural Victoria were less likely to be identified by screening and less likely to receive conservative treatment than their urban counterparts.14
PSA screening and radical prostatectomy for early-stage disease, either independently or in combination, are among the several competing explanations. We emphasise, however, that uncertainty remains about whether PSA screening can reduce mortality, as the results of randomised controlled trials (RCTs) designed to definitively address this issue are not yet available. Also, some commentators have suggested that prostate biopsies on a random sample of asymptomatic men might be as effective as PSA screening, because serum PSA is not related to the small prostate cancers that are currently being diagnosed and treated.12
There is one RCT showing that radical prostatectomy can reduce mortality from prostate cancer in the case of early-stage disease, but there were no differences in all-cause mortality or quality of life.15 Most commentators believe further trials are needed.16 The higher rates of radical prostatectomy in capital cities might reflect better access to urologists.2
Another explanation, perhaps also related to access to urologists, is geographical differences in the management of patients with advanced prostate cancer. For example, the effectiveness of drug-induced androgen deprivation is similar to that of surgical castration,17 and increased use of this more acceptable medical therapy in urban areas might reduce mortality rates from prostate cancer by deferring death sufficiently for competing causes to supervene. Geographical variation in the use of radiation oncology is another possible explanation for the excess of mortality in regional and rural areas. We could not obtain geographical data on use of anti-androgenic drugs or radiation oncology.
Several other studies on trends and differences in prostate cancer mortality and their relationship to PSA testing and radical prostatectomy have produced conflicting results. A study in Austria showed a more rapid decline in mortality from prostate cancer in the Tyrol area, which had a higher rate of PSA screening and early treatment than in the rest of the country.18 However, ecological studies in Seattle, in the United States, and Saskatchewan and British Columbia, in Canada, found no association between screening and mortality.19-21
We found an increasing mortality excess for prostate cancer in regional and rural areas of Australia. Causes of this excess cannot be established by a descriptive study such as this. Nevertheless, based on our analyses, fewer radical prostatectomies in regional and rural areas, perhaps associated with less PSA testing, remain among the several plausible and competing hypotheses. Other possibilities are related to other differences in management, perhaps associated with access to urologists.
At the very least, our results show that the diagnosis and treatment of prostate cancer depends on where the patient lives. Further work is needed to better understand regional differences in cancer treatment and how they might be addressed. A systematic review found only 15 studies (none randomised, only two with a control group, most cross-sectional) which set out to assess the effectiveness of different strategies for delivering cancer services in rural areas.22 There was some evidence suggesting that shared outreach care was safe and made specialist care more accessible to rural patients.
Just as governments and other budget holders need good evidence about the effectiveness of screening and early treatment, they also need good evidence about the best strategies for providing equitable access to services for prostate cancer, and cancer generally, in both urban and rural areas.
1 Details for outcome measures reported in this study
Measure |
Codes |
||||||||||||||
Mortality (1985–2002) |
ICD 10,* C61; ICD 9,† 185 |
||||||||||||||
Incidence (1985–2000) |
ICD 10,* C61; ICD 9,† 185 |
||||||||||||||
Prostate-specific antigen tests (1995/96–2002/03) |
Medicare Item numbers‡ 66655, 66656, 66657, 66658, 66659 |
||||||||||||||
Radical prostatectomy (1995/96–2001/02) |
Procedure codes: ICD 10-AM,§ 37209-00, 37210-00, 37211-00; ICD 9-CM,¶ 60.5 |
||||||||||||||
* International statistical classification of diseases and related health problems, 10th revision. Geneva: World Health Organization, 1992. †International classification of diseases, 9th revision. Geneva: World Health Organization, 1975. ‡Australian Department of Health and Ageing. Medicare benefits schedule. Available at: www.health.gov.au/pubs/mbs (accessed March 2004). §Statistical classification of diseases and related health problems, 10th revision, Australian modification. Sydney: National Centre for Classification in Health, 1998. ¶Australian version of the international classification of diseases, 9th revision, clinical modification. Sydney: National Coding Centre, 1994. |
|||||||||||||||
2 Age-standardised rates per 100 000 men* for prostate cancer mortality and incidence, prostate-specific antigen tests and radical prostatectomy for Australian men residing in capital cities compared with regional and rural areas
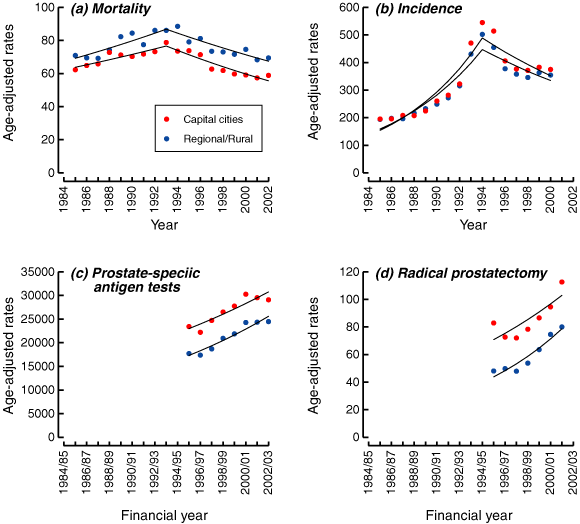
* Age-standardised to Australian 2001 population. Linear trends modelled by means of joinpoint regression.
Received 27 July 2004, accepted 14 December 2004
- Michael D Coory1
- Peter D Baade2
- 1 Health Information Centre, Queensland Health, Brisbane, QLD.
- 2 Viertel Centre for Research in Cancer Control, Queensland Cancer Fund, Spring Hill, QLD.
None identified.
- 1. Strong K, Trickett P, Titulaer I, Bhatia K. Health in rural and remote Australia. Canberra: Australian Institute of Health and Welfare, 2001.
- 2. McCredie M, Bell J, Lee A, Rogers J. Differences in patterns of care of prostate cancer, New South Wales, 1991. Aust N Z J Surg 1996; 66: 727-730.
- 3. US Preventive Services Task Force. Screening for prostate cancer: recommendation and rationale. Ann Intern Med 2002; 137: 915-916.
- 4. Pinnock CB. PSA testing in general practice: can we do more now? [conference report]. Med J Aust 2004; 180: 379-381.
- 5. Australian standard geographic classification. Canberra: Australian Bureau of Statistics. 2003. (ABS Catalogue No. 1216.0).
- 6. Cancer in Australia, 2001. Canberra: Australian Institute of Health and Welfare and Australasian Association of Cancer Registries, 2004.
- 7. Grulich AE, Swerdlow A, dos Santos Silva I, Beral V. Is the apparent rise in cancer mortality in the elderly real? Analysis of changes in certification and coding of cause of death in England and Wales, 1970–1990. Int J Cancer 1995; 63: 164-168.
- 8. Smith DP, Armstrong BK. Prostate-specific antigen testing in Australia and association with prostate cancer incidence in New South Wales. Med J Aust 1998; 169: 17-20.
- 9. Kim HJ, Fay MP, Feuer EJ, Midthume DN. Permutation tests for joinpoint regression with application to cancer rates. Stat Med 2000; 19: 335-351.
- 10. Stata statistical software [computer program]. Release 8.0. College Station, Texas: Stata Corporation, 2003.
- 11. Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics 1985; 41: 55-68.
- 12. Stamey TA, Caldwekk M, McNeal JE, et al. The prostate cancer specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol 2004; 172: 1297-1301.
- 13. Baade PD, Coory MD, Aitken JF. International trends in prostate cancer mortality: the decrease is continuing and spreading. Cancer Causes Control 2004; 15: 237-241.
- 14. Hill DJ, White VM, Giles GG, et al. Changes in the investigation and management of primary operable breast cancer in Victoria. Med J Aust 1994; 161: 110-122.
- 15. Holmberg L, Bill-Axelson A, Helgesen F, et al. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med 2002; 347: 781-789.
- 16. Walsh PC. Surgery and the reduction of mortality from prostate cancer. N Engl J Med 2002; 347: 839-840.
- 17. Chamberlain J, Melia J, Moss S, Brown J. Report prepared for the Health Technology Assessment panel of the NHS Executive on the diagnosis, management, treatment and costs of prostate cancer in England and Wales. Br J Urol 1997; 79(Suppl 3): 1-32.
- 18. Bartsch G, Horninger W, Klocker H, et al. Prostate cancer mortality after introduction of prostate-specific antigen mass screening in the federal state of Tyrol, Austria. Urology 2001; 58: 417-424.
- 19. Lu-Yao G, Albertsen PC, Stanford JL, et al. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ 2002; 325: 740-744.
- 20. Skarsgard D, Tonita J. Prostate cancer in Saskatchewan Canada, before and during the PSA era. Cancer Causes Control 2000; 11: 79-88.
- 21. Coldman AJ, Phillips N, Pickles TA. Trends in prostate cancer incidence and mortality: an analysis of mortality change by screening intensity. CMAJ 2003; 168: 31-35.
- 22. Campbell NC, Ritchie LD, Cassidy J, Little J. Systematic review of cancer treatment programmes in remote and rural areas. Br J Cancer 1999; 80: 1275-1280.





Abstract
Objective: To assess differences in trends for prostate cancer mortality, radical prostatectomy and prostate-specific antigen (PSA) testing for Australian men aged 50–79 years living in capital cities compared with regional and rural areas.
Design: Descriptive, population-based study based on data from official sources from 1985 to the 2002/03 financial year (depending on data availability).
Main outcome measures: Age-standardised rates per 100 000 men aged 50–79 years of mortality from prostate cancer, incidence of prostate cancer, PSA tests and radical prostatectomy.Results: We found a statistically significant and increasing (age-standardised) mortality excess for prostate cancer in regional and rural areas. In 2000–2002 the excess (compared with capital cities) was 21% (95% CI, 14%–29%). Rates of radical prostatectomy in rural and regional Australia were 29% lower (95% CI, 23% lower to 35% lower) than in capital cities. Although PSA testing is common across the whole of Australia, age-standardised rates in 2002/03 were 16% lower (95% CI, 15% lower to 17% lower) in regional and rural areas than in capital cities.
Conclusions: Our results show that the probability of a man having a PSA test and the management of his prostate cancer depend on where he lives. The cause or causes of the prostate cancer mortality excess in regional/rural areas cannot be established in a descriptive study, but fewer radical prostatectomies in regional and rural areas, perhaps associated with less PSA screening, remain among the several competing hypotheses. Other possibilities are related to other differences in management, perhaps associated with access to urologists. Governments and other budget holders need good evidence about the effectiveness of prostate cancer screening and early treatment, but also about the best strategies for providing equitable access to cancer services in both urban and rural areas.