Legionnaires’ disease is an atypical pneumonia caused by Legionella bacteria, often inhaled in contaminated aerosols. Legionella spp. cause an estimated 0.5%–5.0% of cases of community-acquired pneumonia (CAP),1 and up to 15% of cases of CAP requiring hospitalisation.2 Legionella pneumophila serogroup 1 is the major notified cause of legionellosis in the state of Victoria.3 Sources linked to outbreaks worldwide include cooling towers, piped water, fountains and spas.4-8
On 26–27 April 2000, routine follow-up of three notifications of Legionnaires’ disease revealed that all three patients had visited the recently opened Melbourne Aquarium in the 10 days before illness onset. This suggested a source at the Aquarium; environmental investigations were begun immediately. Notifications increased rapidly, becoming Australia’s largest outbreak of Legionnaires’ disease. We describe the outbreak characteristics, investigations into the source of infection, and a case–control study into factors associated with infection.
As this study was part of an outbreak investigation by the Victorian Department of Human Services (VDHS) it did not require formal ethics committee approval. Informed consent was obtained from all participants.
On 27 April 2000, VDHS investigators inspected all possible sources of Legionella organisms at the Melbourne Aquarium. The most likely source was two new air-conditioning cooling towers in the third-floor plant room, fitted with biocide auto-dosing systems and drift eliminators. Cooling-tower water was sampled, then disinfected with chlorine. Water was sampled from all aquarium displays, although none caused significant public exposure to aerosols.
Water samples were sent to the Melbourne Microbiological Diagnostic Unit Public Health Laboratory for isolation of Legionella organisms according to standard methods (AS/NZS 3896: 1998); the limit of detection was 10 colony-forming units (cfu)/mL. L. pneumophila serogroup 1 isolates from the water samples and patients were subtyped using both pulsed-field gel electrophoresis (PFGE) and monoclonal antibody typing techniques. Strain relatedness was assessed using published criteria.9 PFGE was carried out with the CHEF DRIII system (BioRad, Richmond, Va, USA) using restriction endonucleases Xba1 and Sfi1. Biocide and anti-corrosion chemical levels in the water were analysed by the Victorian State Chemistry Laboratory. Cooling tower operation was reviewed for factors possibly contributing to contamination.
In addition to culture, diagnostic tests at the Victorian Infectious Diseases Reference Laboratory (VIDRL) included urinary antigen tests for L. pneumophila serogroup 1 (Binax Legionella Urinary Antigen Enzyme Immunoassay Kit and Binax Legionella NOW immunochromatography, used as per manufacturer-specified methods [Binax Inc, Portland, Ore, USA]) and indirect immunofluorescence assay for serum antibody against L. pneumophila.10
Active case surveillance was initiated by VDHS on 27 April 2000. National and international medical alerts were disseminated because of interstate and international visitors to the Aquarium, and a telephone “hotline” was established.
A case was defined as any person developing fever, cough or pneumonia in the 2 weeks after a visit to the Melbourne Aquarium or its close vicinity, and confirmed by at least one of the following laboratory tests: positive urinary antigen test for L. pneumophila serogroup 1; or a fourfold or greater (to ≥ 128) rise in antibody titre against L. pneumophila between paired acute and convalescent phase sera, or a stable high (> 512) titre in convalescent serum; or isolation of Legionella spp. from respiratory secretions.11
Box 1 shows the details of cases and control participants included in the investigations. Potential control participants were excluded from either study if they reported a clinical diagnosis of pneumonia or any two of the symptoms fever, cough or generalised muscle ache at any time between 11 April and 7 May.
The first case–control study investigated the hypothesis that the Aquarium site was the outbreak source (Box 1). Case exposure data were obtained through routine follow-up, with information sought on recent activities, including visits to the Aquarium and nearby sites. Control participants were obtained from a VDHS computer-assisted telephone interview database of about 10 000 households, previously randomly selected and willing to be surveyed about health issues.12 Control participants included people with silent or unlisted phone numbers, and those interviewed in a language other than English. Interviews were conducted by telephone in June 2000. Control participants were asked about visiting various tourist sites between 11 and 27 April and about symptoms of Legionnaires’ disease in the subsequent 2 weeks.
The second case–control study investigated exposure and patient factors potentially associated with legionellosis among people who visited the Aquarium between 11 and 27 April 2000 (Box 1). Control participants were recruited by systematic selection of phone numbers from the “hotline” call log (from about 7000 calls received, every 10th eligible log entry was chosen after starting at a randomly generated number) and random selection of participants from 126 adult group bookings at the Aquarium during the risk period. A control to case ratio of two was chosen as a compromise between the need for statistical efficiency and what could be achieved quickly and within budget.
Information was collected from case and control participants by telephone interview using a standardised questionnaire. Trained research nurses, blinded to the study hypothesis that specific exposures at the Aquarium increased risk of illness, conducted interviews between 29 May and 14 August 2000.
Blood and urine samples were tested at VIDRL to exclude asymptomatic Legionella infection in control participants (by a single blood sample showing antibody titre < 128 and a negative urinary antigen test). All participants gave informed verbal consent.
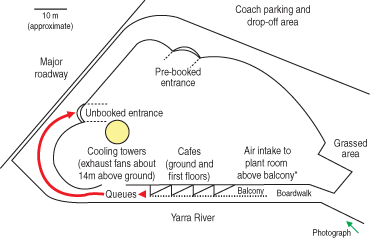
The questionnaire asked about pre-existing illnesses, smoking, and time spent in various locations in and around the Aquarium on the day of visit for both cases and control participants. As the risk period encompassed school holidays, “unbooked” visitors regularly waited in long entry queues from the general “unbooked” entrance, extending along the riverfront and under the balcony of the first floor cafe (Box 2). Standing in this queue or on the balcony was considered a potential exposure risk to contaminated aerosols. “Pre-booked” visitors used a separate entrance (Box 2), avoiding the queue and possibly having different exposure to aerosols.
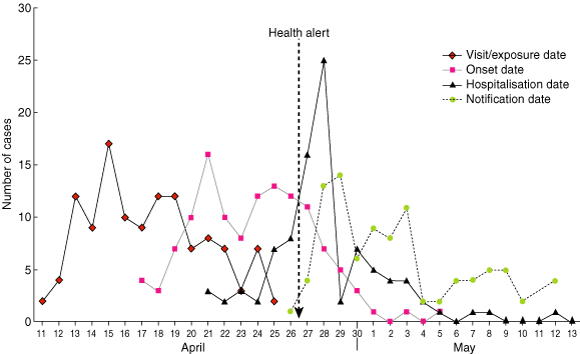
Legionnaires’ disease was confirmed in 110 people who visited the Melbourne Aquarium between 11 and 25 April 2000, including one staff member (Box 3). The daily attack rate varied from 0.36 to 3.0 cases/1000 visitors (4600–6000 visitors/day); the overall attack rate was 1.3 cases/1000 visitors. Another 15 confirmed cases occurred in people who did not visit the Aquarium but were in its immediate vicinity (within 500 metres of the building) during the risk period. Therefore, 125 confirmed cases of Legionnaires’ disease were linked to this outbreak.
Cases were in people aged from 23 to 89 years (median, 64 years); 57% were men. The average incubation period was 6.3 days (median, 6 days; range, 1–16 days). Patients who were hospitalised (76%) were significantly older than those not hospitalised (64.4 v 55.1 years; P = 0.01). The overall case–fatality ratio was 3.2% (4 deaths: 2 visitors, 2 passers-by).
The earliest illness onset was on 17 April and the first notification was on 26 April (Box 3). The outbreak was recognised the following day, and control measures were implemented immediately, 10 days after the earliest onset. By 27 April, 77% of patients were ill, but only 28% of those eventually hospitalised had been admitted.
Most cases (83%) were diagnosed by a positive urinary antigen test, including all 11 culture-positive cases; few samples were submitted for culture. Serological testing was the sole basis of diagnosis for 17% of cases.
The first case–control study showed that, of 129 people with Legionnaires’ disease notified in Victoria with onset between 11 April and 9 May, 100 (78%) had visited the Aquarium, compared with only five of 305 (1.6%) asymptomatic community control participants (Box 1). This confirmed a very strong association between being notified to VDHS with Legionnaires’ disease and visiting the Aquarium between 11 and 27 April (odds ratio [OR], 207; 95% CI, 73–630).
The second case–control study included 104 case and 201 control participants in the analysis (Box 1). The 53 control participants who were excluded did not differ from the 201 included in age and sex distribution or total time spent at the Aquarium. Control participants were frequency-matched to cases by age and sex, but, with insufficient older male control participants, male case participants were significantly older than male control participants (61.6 v 54.4 years; P = 0.004). These residual differences between cases and controls were accounted for by multivariable analysis.
No visit exposure characteristics were significant risk factors on univariate analysis, although “standing in a queue or waiting outside” neared significance (P = 0.09; OR, 1.54; 95% CI, 0.94–2.50). There was no association between illness and going onto the balcony (OR, 0.97; 95% CI, 0.56–1.67), although 33% of hospitalised patients went onto the balcony compared with 4% of non-hospitalised patients (P = 0.002). The only personal characteristic significantly associated with disease on univariate analysis was smoking (Box 4).
Factors were considered for the multivariable analysis based on preliminary univariate analysis and biological and environmental plausibility. The results from the best model to explain the study data (Box 5) were obtained by eliminating all two-factor interactions and main effects that were not statistically significant at the 5% level. Factors used for frequency matching were retained. Smoking was an independent dose-dependent risk factor. In addition, there was effect-measure modification between which entrance a visitor used and whether or not they queued or waited before entry (P = 0.02), as well as between queuing and sex (P = 0.02), but not entrance and sex (P = 0.4), and not all three terms together (P > 0.9). Because of these complex interactions, odds ratios in relation to these terms are expressed as the eight combinations of the three variables.
Legionnaires’ disease was confirmed in 125 people who visited or passed within 500 m of the Melbourne Aquarium between 11 and 27 April 2000. There was a very strong association between visiting the Aquarium in the risk period and contracting Legionnaires’ disease (OR, 207; 95% CI, 73–630). The outbreak was linked to contaminated air-conditioning cooling towers at the Aquarium. The climatic variability in Melbourne, particularly in autumn, often results in sporadic functioning of air-conditioners on autothermostats, facilitating growth of contaminants in water-cooled air-conditioning systems and subsequent dispersion.
Both cooling towers were contaminated with Legionella organisms and did not contain detectable biocide when sampled on 27 April 2000, indicating no effective disinfection of the cooling-tower water. This was found to be most likely due to a faulty dosing pump and lack of regular inspection of the system. Under these circumstances, despite the presence of modern drift eliminators, aerosols containing Legionella organisms were dispersed in the environment. Contaminated aerosols were inhaled by people in the vicinity of the Aquarium, leading to Legionnaires’ disease in those who were susceptible. This outbreak occurred before the introduction to Victoria in 2001 of risk-based Legionella legislation, which requires high-level maintenance of all water-cooled air-conditioning systems.
Rapid notification of cases, identification of the geographical link, and implementation of control measures prevented further infections occurring after 27 April (Box 3). Rapid distribution of health alerts may have prompted infected people to seek treatment early in their illness, and doctors to test rapidly for Legionella infection in Aquarium visitors presenting with even mild symptoms.8,15,16 This may have contributed to the relatively low hospitalisation and mortality rates compared with other outbreaks with possibly slower recognition of the outbreak source.5,7,17,18 Australian antibiotic guidelines advise erythromycin for treatment of severe CAP and legionellosis; empirical use of erythromycin may also have helped reduce morbidity.
Isolation of Legionella organisms from a clinical specimen is the gold standard diagnostic test, and allows for molecular subtyping2 and comparison with environmental isolates. However, samples for the urinary antigen test are easier to obtain and yield results within hours rather than days.19 Alerts and widespread urinary antigen testing may have revealed a wider spectrum of disease than might otherwise have been detected, altering apparent morbidity and mortality rates.20 The proportion of non-hospitalised cases in this outbreak (24%), similar to a recent Spanish outbreak,21 may indicate there is a proportion of milder disease that is not diagnosed in non-outbreak settings. Historically, 94%–98% of notified cases in Victoria have been hospitalised,19 similar to hospitalisation rates for sporadic cases in the 1970s.22
The second case–control study sought to determine risk factors associated with Legionnaires’ disease in Aquarium visitors. The multivariable model included age, smoking status, and the combination of sex plus entrance used plus queuing (Box 5). Widely reported risk factors for Legionnaires’ disease include increasing age, male sex, smoking, heavy alcohol intake and chronic illness (eg, diabetes mellitus, chronic lung disease, renal disease, malignancy, immunocompromised status).1,17,18,23 The risk factors found in outbreaks and sporadic community-acquired Legionnaires’ disease may differ, and, indeed, investigations may match cases and control participants on some “known risk factors”.24,25 Our case–control study could not assess sex and age as independent risk factors because of frequency matching. However, only 57% of cases were men, at odds with the striking male preponderance reported previously.21,22,26
Some accepted risk factors, in particular chronic illness, were not significant in our case–control study, although the results were consistent with an increased risk (up to twofold) among patients with chronic illness (eg, “seeing a doctor regularly for lungs, heart or diabetes”). A study of a recent outbreak in Spain also found predisposing factors, including chronic illness, were not significant.21 An early case–control study in the United States found underlying illness to be a relatively small and non-specific predisposing factor for community-acquired Legionnaires’ disease.23 The lack of significant association in our case–control study between Legionnaires’ disease and chronic illness might be due to inaccurate self-report or detection of milder illness compared with some previous investigations. Some factors may be significant risks for only the more severe end of the disease spectrum. Separate analysis of hospitalised and non-hospitalised patients in this outbreak is problematic, as early intervention by VDHS may have influenced outcomes.
A characteristic considered a risk factor for Legionnaires’ disease, and confirmed in our case–control study, was smoking. Odds of illness among current smokers increased in a dose-dependent manner, with those smoking up to 70 cigarettes per week showing a lesser increase (OR, 4.0) than those smoking more than 70 per week (OR, 13.5) (Box 5). An increase in CAP risk has been associated with smoking status, number of cigarettes smoked per day, and lifetime smoking.27 A previous case–control study of sporadic community-acquired Legionnaires’ disease found an increased risk of illness associated with an increasing number of cigarettes currently smoked per day.23 Our case–control study has shown the dose-dependent effect of smoking on this form of CAP in a large outbreak setting with a wide spectrum of disease severity.
The statistical interaction of “entrance” and “queue” exposures was not unexpected, as the intention of pre-booking (thus using the “pre-booked” entrance) was to minimise queuing time. However, the interaction of “queue” with sex was unexpected. The interactions mean that the three exposures involved (sex plus entrance plus queuing) must be considered together, but interpretation is difficult (Box 5). The differences in odds ratios for the groups cannot be explained by age or smoking behaviour, as these factors were not additional effect modifiers in the model. Overall, queuing or waiting outside the building posed a greater risk than not doing so, with waiting at the “pre-booked” entrance a greater risk than queuing at the “unbooked” entrance. For reasons that are unclear, the one exception to the increased risk from queuing was that the greatest risk of illness (OR, 7.1) was in men who used the “unbooked” entrance and did not queue.
A dose–response effect between time (frequency and duration) spent in close proximity to a contaminated cooling tower and the likelihood of having Legionnaires’ disease has been found previously.24 In our case–control study there was no association between disease and time spent at various locations inside or outside the Aquarium. The greater proportion of hospitalised compared with non-hospitalised patients who went onto the balcony indicates that, while this was not associated with contracting disease, it may have influenced illness severity. Infection of people walking past the building, together with the lack of association between duration and site of exposure, suggests that transient exposure was sufficient to cause infection.
A limitation of this study was possible sampling bias among control participants; this was unavoidable and unmeasurable, as limited sources of visitor control participants were available in the midst of a very sensitive public investigation.
This outbreak provided a unique opportunity to investigate risk factors for a wide spectrum of Legionnaires’ disease using a case–control study. Historically reported risks not found to be significant in this outbreak, such as chronic illness, may be risk factors for only the more severe cases of Legionnaires’ disease that have been detected in the past. The ability to detect outbreaks rapidly using the urinary antigen test may change our understanding of the epidemiology of Legionnaires’ disease. This outbreak stimulated new legislation which requires registration of cooling towers linked to risk management plans.
1: Numbers of cases and controls for outbreak and ca se–control studies of Legionnaires’ disease (LD)
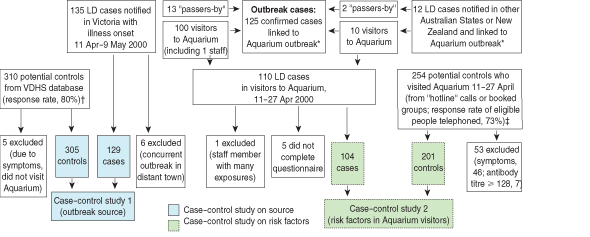
VDHS = Victorian Department of Human Services * Case linked to Aquarium outbreak defined as patient visited or passed by Aquarium, 11–27 Apr 2000.
† Frequency matched to cases by sex and 5-year age group.
‡ Frequency matched to cases by sex and 10-year age group.
3: Confirmed cases of Legionnaires’ disease, by date of visit, onset of illness, case notification and hospital admission
4: Univariate analysis of patient risk factors for Legionnaires’ disease in visitors to the Aquarium*
Variable |
Cases (n = 104) |
Controls (n = 201) |
Odds ratio (95% CI)† |
P † |
|||||||
Seeing a doctor regularly |
65 |
119 |
1.15 (0.71–1.87) |
0.6 |
|||||||
Seeing a doctor for a problem of lungs, heart or diabetes |
38 |
60 |
1.35 (0.82–2.23) |
0.2 |
|||||||
Smoking |
|
|
|
|
|||||||
Never |
34 |
106 |
1.00 |
|
|||||||
Quit > 20 years ago |
10 |
28 |
1.11 (0.49–2.53) |
|
|||||||
Quit ≤ 20 years ago |
26 |
47 |
1.72 (0.93–3.19) |
|
|||||||
Currently smoke ≤ 70 cigarettes/week |
12 |
10 |
3.74 (1.49–9.42) |
|
|||||||
Currently smoke > 70 cigarettes/week |
22 |
10 |
6.86 (2.96–15.9) |
< 0.001 |
|||||||
Drink alcohol |
73 |
160 |
0.60 (0.35–1.04) |
0.07 |
|||||||
* Responses > 95% for the above variables were classifiable and are included in the table. † For categorical variables with more than two categories, odds ratios of categories are reported in relation to a baseline category and the P value is for the overall test that all of the true odds ratios are equal to 1. |
|||||||||||
5: Multivariable analysis of risk factors for Legionnaires’ disease in visitors to the Aquarium*
Predictor constant |
Cases (n = 104) |
Controls (n = 201) |
Odds ratio (95% CI) |
P ‡ |
|||||||
Age |
|
|
|
|
|||||||
< 50 years |
17 |
61 |
1.00 |
< 0.001 |
|||||||
50–64 years |
35 |
69 |
3.32 (1.47–7.50) |
|
|||||||
≥ 65 years |
52 |
71 |
6.12 (2.67–14.1) |
|
|||||||
Smoking |
|
|
|
|
|||||||
Never smoked |
34 |
106 |
1.00 |
< 0.001 |
|||||||
Quit > 20 years ago |
10 |
28 |
0.75 (0.31–1.81) |
|
|||||||
Quit ≤ 20 years ago |
26 |
47 |
1.63 (0.83–3.19) |
|
|||||||
Currently smoke ≤ 70 cigarettes/week |
12 |
10 |
4.02 (1.47–11.0) |
|
|||||||
Currently smoke > 70 cigarettes/week |
22 |
10 |
13.50 (5.01–36.5) |
|
|||||||
Interacting risk factors |
|
|
|
|
|||||||
Female, pre-booked,* no queue† |
5 |
27 |
1.00 |
|
|||||||
Female, unbooked,* no queue† |
10 |
29 |
2.56 (1.05–6.20) |
|
|||||||
Male, pre-booked, no queue |
8 |
22 |
2.78 (1.17–6.61) |
|
|||||||
Male, unbooked, queue |
23 |
46 |
3.05 (1.09–8.54) |
|
|||||||
Female, unbooked, queue |
27 |
42 |
3.96 (1.49–10.6) |
|
|||||||
Male, pre-booked, queue |
8 |
12 |
5.18 (1.65–16.3) |
|
|||||||
Female, pre-booked, queue |
8 |
7 |
6.72 (2.10–21.5) |
|
|||||||
Male, unbooked, no queue |
14 |
15 |
7.10 (1.93–26.1) |
|
|||||||
* Pre-booked individuals entered through the entrance for pre-booked groups. Unbooked individuals entered via the general entrance. † Queue = individual queued or waited outside the building before entry. ‡ P value is for the overall test that all of the odds ratios in a section are equal to 1. |
|||||||||||
- Jane E Greig1
- John A Carnie2
- Graham F Tallis3
- Bernard Zwolak4
- William G Hart5
- Charles S Guest6
- Norbert J Ryan7
- Jennie A Leydon8
- Agnes G Tan9
- Ian R Gordon10
- 1 Department of Human Services, Melbourne, VIC.
- 2 National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT.
- 3 Victorian Infectious Diseases Reference Laboratory, Melbourne, VIC.
- 4 University of Melbourne, Melbourne, VIC.
There were no external sources of funding. Assistance in the outbreak investigation: J Bowman and D Lightbody (Environmental Health Unit, VDHS); M Moloney, A Murphy, P Lynch, J Gregory, and P Robinson (Communicable Diseases Section, VDHS); J Li (National Centre for Epidemiology and Population Health); G Hogg, H Kelly, and H Karunajeewa (VIDRL). Case–control study interviews: S Smith, E McGrath, S Proctor, S Palmer, G Neave, and G Simmons. Advice on the planning of the case–control study and/or this manuscript: M Kirk (Communicable Diseases Section, VDHS); M Beers (National Centre for Epidemiology and Population Health); J McAnulty (New South Wales Communicable Diseases Surveillance and Control Branch); R Hall (South Australian Communicable Disease Control Branch).
None identified.
- 1. Chin J, ed. Control of communicable diseases manual. 17th ed. Washington, DC: American Public Health Association; 2000.
- 2. Formica N, Tallis G, Zwolak B, et al. Legionnaires’ disease outbreak: Victoria’s largest identified outbreak. Commun Dis Intell 2000; 24: 199-202.
- 3. Department of Human Services. Surveillance of notifiable infectious diseases in Victoria 1999. Melbourne: DHS, 2000.
- 4. Breiman RF. Modes of transmission in epidemic and nonepidemic Legionella infection: directions for further study. In: Barbaree J, Breiman R, Dufour A, eds. Legionella. Current status and emerging perspectives. Washington, DC: American Society for Microbiology, 1993: 30-35.
- 5. Levy M, Westley-Wise V, Blumer C, et al. Legionnaires’ disease outbreak, Fairfield 1992: public health aspects. Aust J Public Health 1994; 18: 137-143.
- 6. Hoebe CJPA, Kool JL. Control of Legionella in drinking-water systems. Lancet 2000; 355: 2093-2094.
- 7. Den Boer JW, Yzerman EPF, Schellekens J, et al. A large outbreak of Legionnaires’ disease at a flower show, the Netherlands, 1999. Emerg Infect Dis 2002; 8: 37-43.
- 8. Jernigan DB, Hofmann J, Cetron M, et al. Outbreak of Legionnaires’ disease among cruise ship passengers exposed to a contaminated whirlpool spa. Lancet 1996; 347: 494-499.
- 9. Tenover F, Arbeit R, Goering R. How to select and interpret molecular strain typing methods for epidemiological studies of bacterial infections: a review for healthcare epidemiologists. Molecular Typing Working Group of the Society for Healthcare Epidemiology of America. Infect Control Hosp Epidemiol 1997; 18: 426-439.
- 10. Harrison T, Taylor A. The diagnosis of Legionnaires’ disease by estimation of antibody levels. In: Harrison T, Taylor A, eds. A laboratory manual for Legionella. Chichester, UK: John Wiley and Sons, 1988: 113-135.
- 11. Communicable Diseases Network Australia. Interim surveillance case definitions for the Australian National Notifiable Diseases Surveillance System. Version 1. Canberra: Department of Health and Ageing, 2004.
- 12. Victorian Population Health Survey 1999. A demonstration survey. Melbourne: Victorian Government Department of Human Services, 2000. Available at: www.dhs.vic.gov.au/phd/healthsurveillance/downloads/vphs1999.pdf (accessed Oct 2003).
- 13. Epi Info. Version 6.04. Atlanta, Ga: Centers for Disease Control and Prevention, 1997.
- 14. MINITAB. Version 13. State College, Pa: Minitab Inc, 2003.
- 15. Buising K, O’Reilly M, Paull A, et al. Legionella pneumophila: not just pneumophila. Med J Aust 2001; 174: 476-477.
- 16. Frankel DH. Urine test identifies Legionnaires’ outbreak. Lancet 1996; 348: 1229.
- 17. Kociuba KR, Buist M, Munro R, et al. Legionnaires’ disease outbreak in south western Sydney, 1992. Clinical aspects. Med J Aust 1994; 160: 274-277.
- 18. Hoge C, Breiman R. Advances in the epidemiology and control of Legionella infections. Epidemiol Rev 1991; 13: 329-340.
- 19. Formica N, Yates M, Beers M, et al. The impact of diagnosis by Legionella urinary antigen test on the epidemiology and outcomes of Legionnaires’ disease. Epidemiol Infect 2001; 127: 275-280.
- 20. Bell JC, Jorm LR, Williamson M, et al. Legionellosis linked with a hotel car park — how many were infected? Epidemiol Infect 1996; 116: 185-192.
- 21. Garcia-Fulgueiras A, Navarro C, Fenoll D, et al. Legionnaires’ disease outbreak in Murcia, Spain. Emerg Infect Dis 2003; 9: 915-921.
- 22. England AC, Fraser D, Plikaytis B, et al. Sporadic legionellosis in the United States: the first thousand cases. Ann Intern Med 1981; 94: 164-170.
- 23. Storch G, Baine W, Fraser D, et al. Sporadic community-acquired Legionnaires’ disease in the United States. A case-control study. Ann Intern Med 1979; 90: 596-600.
- 24. Brown CM, Nuorti PJ, Breiman RF, et al. A community outbreak of Legionnaires’ disease linked to hospital cooling towers: an epidemiological method to calculate dose of exposure. Int J Epidemiol 1999; 28: 353-359.
- 25. Straus WL, Plouffe JF, File TM, et al. Risk factors for domestic acquisition of legionnaires disease. Arch Intern Med 1996; 156: 1685-1692.
- 26. Broome C, Fraser D. Epidemiologic aspects of legionellosis. Epidemiol Rev 1979; 1: 1-16.
- 27. Almirall J, Gonzalez C, Balanzo X, et al. Proportion of community acquired pneumonia cases attributable to tobacco smoking. Chest 1999; 116: 375-379.






Abstract
Objective: To investigate the source and risk factors associated with Australia’s largest outbreak of Legionnaires’ disease.
Design and setting: Epidemiological and environmental investigation of cases of Legionnaires’ disease associated with visits to the Melbourne Aquarium; two case–control studies to confirm the outbreak source and to investigate risk factors for infection, respectively.
Participants: Patients with confirmed Legionnaires’ disease who visited the Melbourne Aquarium between 11 and 27 April 2000 were compared (i) with control participants from the community, and (ii) with control participants selected from other visitors to the Aquarium during this period.
Main outcome measures: Risk factors for acquiring Legionnaires’ disease.
Results: There were 125 confirmed cases of Legionnaires’ disease caused by Legionella pneumophila serogroup 1 associated with the Aquarium; 76% of patients were hospitalised, and four (3.2%) died. The Aquarium cooling towers were contaminated with this organism. Visiting the Aquarium was significantly associated with disease (odds ratio [OR], 207; 95% CI, 73–630). The case–control study indicated that current smoking was a dose-dependent risk (multivariable OR for currently smoking > 70 cigarettes/week, 13.5; 95% CI, 5–36), but chronic illness and duration of exposure at the site were not significant risks.
Conclusions: This study showed an association between poorly disinfected cooling towers at the Aquarium and Legionnaires’ disease in visitors, and confirmed current smoking as a critical risk factor. The rapid response, publicity, and widespread urinary antigen testing may have resulted in detection of milder cases and contributed to the relatively low apparent morbidity and mortality rates. The urinary antigen test allows rapid identification of cases and may be changing the severity of illness recognised as Legionnaires’ disease and altering who is considered at risk.