Although the completed phase of the Human Genome Project has provided the entire human DNA sequence, the next challenge is to determine the functions of most of the ~35 000 genes in the human genome. Each day, researchers discover the functions of new genes and increase the knowledge that can be translated into clinical practice. This growth in knowledge fuels, in turn, the expansion of DNA testing both for diagnosis and for prediction of disease susceptibility. Clinicians, whether in general practice or in other specialties, need to accommodate the "new genetics", with its focus on DNA variation and its role in disease.
In the new genetics that has emerged from DNA-based information, the doctor–patient relationship becomes more complex, as knowledge of an individual's DNA status has health implications not only for the individual, but also for the family. The management of a genetic disorder is likely to involve family members, making follow-up and counselling potentially more difficult logistically, and to evoke ethical issues, such as the privacy of the individual versus the potential benefits to family members. General practitioners are at the "coalface" of the new genetics because of their close links with the patient and involved families.
When a genetic condition can be detected but no treatment is available, long term management and follow-up can unleash a constellation of medical, psychosocial and reproductive issues. This can be illustrated by apolipoprotein E (APOE) genetic tests, which can predict elevated risks for common conditions such as coronary artery disease or Alzheimer's disease.1 In this environment, the GP and other specialists are all key players with their particular in-depth knowledge of the medical issues.
The challenges for doctors will be finding the time and commitment to deal with the inexorable changes in clinical practice as the new genetics becomes increasingly involved in all areas of medicine. Counselling and ethical issues related to familial disease require new skills, and more resources. Increasingly, GPs and specialists, including clinical geneticists and genetic counsellors, will need to work as part of a molecular medicine (DNA) team to provide the necessary range of skills.
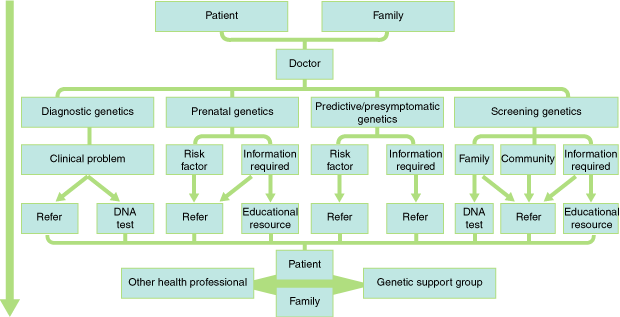
The impact of the new genetics on clinical practice is highlighted in the Box, which details clinical decision pathways in four different management spheres of the new genetics: diagnostic, prenatal, predictive/presymptomatic and screening genetics. The potential directions in these decision mazes are explored in the following scenarios.
In this scenario, a patient presents with clinical features of a disorder, and a DNA test is undertaken to confirm a diagnosis. For example, diabetes, altered skin pigmentation and a persistently raised ferritin level would be consistent with haemochromatosis. Confirmation would previously have required liver biopsy, but an alternative, non-invasive option with the new genetics is a DNA test.2 There is a question of whether the GP should refer to a specialist, or independently initiate the DNA test, which looks for the common mutation C282Y (Box).2 Initiating the test is reasonable if the GP understands the implications of the DNA test result, including the significance of a negative result. The GP also needs to understand the test's dependence on the ethnic background of the person being tested. C282Y is predominantly found in people with a north-western European background. Failure to find C282Y in an individual of Asian or Mediterranean background is less helpful, as the predominant mutation in these populations is H63D, and the significance of this mutation in terms of developing clinical haemochromatosis remains unclear.2,3 The genetic laboratory can provide a result, but cannot be expected to give a definitive interpretation of the result's significance without adequate clinical information as to why the test was ordered.
Once the clinical diagnosis is confirmed, the patient can be treated, but the management should also include family members at risk, who will also need counselling. If agreeable, at-risk family members might need DNA testing to predict the future development of a genetic disorder. In these circumstances a key question is who will be responsible for the family testing and counselling. Will it be the specialist, the GP or the genetic counselling service now available in most public hospitals in Australia and in outreach clinics in rural centres? We propose that a team approach in diagnostic genetics will produce the best result for the patient and family, as it will combine the clinical and laboratory expertise (specialists), the genetics knowledge (genetics clinic) and the long term care of the patient and family (GP).
Two possible prenatal testing scenarios exist. The first involves a pregnancy that has high risk for a genetic disorder or fetal abnormality. This risk is identified because of a family history, as when one of the partners is a known carrier of a disease such as cystic fibrosis, or because of advanced maternal age, with its associated risk of a chromosomal disorder such as Down syndrome. People in these situations are often best referred to a specialist genetics clinic or fetal medicine unit, where the risk can be accurately determined and the pregnancy managed accordingly (Box).
The second prenatal testing scenario is when couples wishing to start a family make a general inquiry about risks, or procedures available to monitor their pregnancy. This scenario includes carrier testing for thalassaemia, maternal serum screening using biochemical markers to identify conditions such as Down syndrome or neural tube defects, as well as first-trimester ultrasound studies for Down syndrome or structural abnormalities. Couples with no specific risk factors but wanting information could be managed by GPs or obstetricians who have the necessary knowledge and counselling skills.
Predictive or presymptomatic DNA testing allows genetic disorders to be detected in advance of clinical presentation. This ability to predict disease development before symptoms or signs occur is a particularly powerful new option. For example, offspring of a parent with familial adenomatous polyposis (FAP) have a 50% chance of inheriting the abnormal gene. Once the parental FAP DNA mutation is known, the offspring can have a DNA test to determine if they have inherited the normal parental gene (ie, they are no longer at risk) or the gene with the DNA mutation (ie, they will definitely develop FAP at some future date). In the latter case, the onset of the disease can be predicted long before there are any clinical features, and with it the associated risk for colon cancer. On the other hand, predictive genetics can lead to adverse psychosocial consequences if the individual or the family cannot cope with this predictive information. Furthermore, there is the possibility of discrimination, as the individual is labelled with a disease that has yet to develop, or indeed might never develop. High profile examples of predictive testing include BRCA1 and BRCA2 genes for breast cancer, and genes for a number of serious and non-treatable adult-onset neurological disorders, such as Huntington disease.4,5 The haemochromatosis scenario also involves predictive genetics, but is less emotive, as there is a relatively simple intervention (regular venesection) to prevent or treat this condition.
Because patients undergoing predictive DNA testing have no symptoms or clinical signs, it is particularly important that this type of DNA testing is undertaken appropriately, that counselling implications are addressed before testing, and that support services are available afterwards.6 Getting the answer wrong either way has long-term implications which could lead to inappropriate lifestyle decisions for the patient and family, and distress when the truth belatedly becomes known. Because of the complexities associated with predictive testing, the requirement for experienced and intensive counselling and the necessity, in some cases, for ongoing support, it is essential that tests predictive of serious diseases (such as breast cancer or Huntington disease) be initiated through a clinical genetics service that has appropriate skills and resources. The Box recommends referral for all predictive DNA tests, although there will be exceptions. Examples of tests that might not require referral are haemochromatosis or predisposition to thrombosis through the Factor V Leiden DNA mutation.2,7 A medical practitioner who deals with the clinical consequences of these disorders should be well placed to interpret the DNA test result (taking into consideration interacting environmental and other genetic factors), and be able to explain that not all people testing positive will develop the disorder, particularly if appropriate interventions are followed. The caveat to this is that the medical practitioner is sufficiently trained in both basic knowledge and counselling skills required for this level of predictive genetic testing.
In this scenario, there are two broad screening strategies: family and community.
An example of family screening is DNA testing for cystic fibrosis carrier status. The carriers are completely healthy, and the implications of a positive test result are relevant only for decisions involving reproduction. Testing might be offered to close relatives at high risk, or to the wider family.
Community screening has many variations. For example, screening can be based on increased predisposition because of ethnic background (as for thalassaemia in people of Mediterranean, Middle Eastern or Asian origin, or Tay–Sachs disease for Ashkenazi Jews). In other cases, community screening could identify people with a gene mutation to establish a population-based prevention program. An example would be testing for haemochromatosis in the general population with a view to preventing the disease by prophylactic venesection.8 A third example of community screening is the newborn screening program, which tests for common or preventable diseases that require early treatment, such as phenylketonuria (PKU) and hypothyroidism.
Family screening can be arranged through GPs or specialists, depending on their knowledge and the complexity of the underlying disorder (Box). On the other hand, the value of community screening varies from case to case, and consent is a more complex issue. For example, PKU screening is universal in developed countries and has saved countless individuals from intellectual handicap, whereas community screening for haemochromatosis is still controversial.2,3 Many individuals and families, prompted by media stories, will approach their GP to inquire about genetic testing, making the healthcare professional an important "gatekeeper" for genetic knowledge. The GP will have appropriate resources to answer some questions, but in other cases should refer to the specialist or make use of other healthcare professionals and genetic support groups.
The new DNA-based genetics will have an increasing impact on medical practice. Few doctors currently understand the complexities involved in counselling and in interpreting DNA tests, or the rapidly moving field of genetics that is technology-driven and relies on computer-based knowledge. This might lead to a suggestion that the new genetics can only be adequately handled by experts within a clinical genetics unit where there will be the skills to ensure that patients (and their families) undergo appropriate DNA testing, with the best outcomes. However, this option has drawbacks. Many diseases with a genetic component to aetiology, such as cancer, heart disease and haemochromatosis, are very common, and resources in clinical genetics units are limited. Most importantly, long term medical management and support of the patient and family may well be compromised if the GP and specialist are not directly involved in offering care based on this new and powerful means of diagnosis.
It is unrealistic to expect every doctor to have the knowledge, time or resources to deal with all aspects of the new genetics. This is an opportunity for the colleges or universities to devise programs that allow interested doctors to seek additional training. This skill would need to be recognised, including a financial incentive to ensure that the time commitment required for genetic counselling and family follow-up is available. In this way, a cohort of GPs and specialists can take their place in the molecular medicine (DNA) team rather than simply functioning as a source of referral. In 1999, new Medical Benefits Schedule items were introduced to enhance GPs' ability to coordinate more complex care of patients such as might occur with genetic counselling. However, this has not proven to be very successful,9 and more innovative ways need to be considered.
In this brief overview of the new genetics, we have focused on DNA technology and its impact on clinical practice. The overview was given to several healthcare professionals who were invited to provide more substantial articles on the various issues related to the new genetics. We hope that the articles in this series will prove informative, and a focus for debate. This is a timely series, as, in 2003, the joint Australian Law Reform Commission/National Health and Medical Research Council Australian Health Ethics Committee Enquiry into the Protection of Human Genetic Information will deliver to the federal Attorney General and the federal Minister for Health recommendations that will have far-reaching effects for clinical practice and the new genetics.
- Ronald J A Trent1
- Robert Williamson2
- Grant R Sutherland3
- 1 Department of Molecular and Clinical Genetics, Royal Prince Alfred Hospital, Camperdown, NSW.
- 2 Murdoch Children's Research Institute, University of Melbourne, Royal Children's Hospital, Parkville, VIC.
- 3 Department of Cytogenetics and Molecular Genetics, Women's and Children's Hospital, Adelaide, SA.
None identified.
- 1. Apolipoprotein E; APOE. MIM Number 107741. Online Mendelian Inheritance in Man (OMIM). Baltimore MD: Johns Hopkins University. Last edited 6 Sep 2002. Available at: http://www.ncbi.nlm.nih.gov/omim/ (accessed Mar 2003).
- 2. Powell LW. Hereditary hemochromatosis and iron overload diseases. J Gastroenterol Hepatol 2002; 17: S191-S195.
- 3. Robson KJH, Merryweather-Clarke AT, Pointon JJ, et al. Diagnosis and management of haemochromatosis since the discovery of the HFE gene: a European experience. Br J Haematol 2000; 108: 31-39.
- 4. Culver JO, Hull JL, Levy-Lahad E, et al. BRCA1 and BRCA2 hereditary breast/ovarian cancer. Updated 4 March 2000. In: GeneReviews at GeneTests. GeneClinics: Medical Genetics Information Resource (database online). Seattle: University of Washington, 1997–2002. Available at: http://www.geneclinics.org (accessed Dec 2002).
- 5. Goizet C, Lesca G, Durr A. Presymptomatic testing in Huntington's disease and autosomal dominant cerebellar ataxias. Neurology 2002; 59: 1330-1336.
- 6. Lloyd FJ, Reyna VF, Whalen P. Accuracy and ambiguity in counseling patients about genetic risk. Arch Intern Med 2001; 161: 2411-2413.
- 7. Press RD, Bauer KA, Kujovich JL, Heit JA. Clinical utility of factor V Leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med 2002; 126: 1304-1318.
- 8. Yapp TR, Eijkelkamp EJ, Powell LW. Population screening for HFE-associated haemochromatosis: should we have to pay for our genes? Intern Med J 2001; 31: 48-52.
- 9. Harris MF. Case conferences in general practice: time for a rethink? Med J Aust 2002; 177: 93-94. <MJA full text>





Abstract
A "new genetics" has emerged driven by knowledge gained at the DNA level.
In clinical practice, a practical application of the new genetics is DNA testing, which can be expected to expand with the completion of the Human Genome Project as the functions of new genes are discovered.
Genetic DNA testing scenarios include diagnostic DNA testing, prenatal DNA testing, predictive (presymptomatic) DNA testing and screening DNA testing.
The challenge for genetic DNA testing and clinical practice will be to define the roles to be played by the general practitioner, the specialist, and other healthcare professionals.
From the patients' and families' perspective, the new genetics will best be implemented if a planned approach is adopted in the ordering of DNA tests and the associated counselling and support processes.