In asthma, taking inhaled corticosteroids (ICS) substantially improves morbidity and mortality,1 and is the basis of pharmacotherapy for disease control.2,3 However, there is great variability in the doses of ICS prescribed for asthma. While the Australian guidelines recommend a specific dose for adults (eg, 500 μg of fluticasone per day), the recent Global Initiative for Asthma (GINA) guidelines recommend a wide dose range from 200 μg to 1000 μg of beclomethasone per day.3 There is now evidence that most (> 90%) of the benefit from ICS is achieved at relatively low doses, equivalent to 250 μg of fluticasone per day.4 Despite this, very high doses of ICS continue to be used, particularly in Australia and New Zealand,5 and there are emerging reports of significant side effects occurring with high dose ICS use.6
We sought to evaluate the balance between the efficacy and safety of different doses of ICS for asthma, and to communicate this to prescribers in an efficient way using the evidence-based measures of number needed to treat (NNT — the number of patients required to receive an intervention for an additional patient to benefit) and number needed to harm (NNH — the number of patients needed to receive an intervention for an additional patient to develop a side effect).7
Level 1 evidence8 of the efficacy and safety of different doses of ICS in asthma was identified by searching the Cochrane Database of Systematic Reviews9 using the search terms "asthma or wheez* and inhaled corticoster* or beclometh* or triamcin* or flutic* or budes* or betameth* or flunis* or cicles* or momet*". Six completed systematic reviews fitted the criteria (ie, compared ICS with either placebo or different doses of ICS for chronic asthma).10-15 Data were extracted from the results of meta-analyses included in the reviews.
Estimates of efficacy of ICS based on several parameters (FEV1, peak expiratory flow, night waking and rescue β2-agonist use) were calculated using the weighted mean difference or standardised mean difference from the meta-analyses for each dose of ICS compared with placebo.10 Insufficient data were available for efficacy estimates of fluticasone 2000 μg.
NNT and NNH were calculated for each dose of ICS using the odds ratios and control-group event rates from the meta-analyses. The method of calculating NNT is given in Box 1. The randomised controlled trials used standard predefined criteria to report the number of subjects withdrawn because of poor asthma control or worsening asthma and development of side effects.
Beclomethasone and budesonide were found to be superior to placebo in efficacy in terms of symptoms, lung function and exacerbations.12,14 However, dose–response effects could not be adequately evaluated, as the relatively small number of trials assessing a wide range of doses limited the ability to aggregate results.13,15 Adequate data were available for evaluation of the dose–response effects for fluticasone in asthma.10,11 These results are displayed graphically in Box 2 A–D. Clinically small, but occasionally statistically significant, dose–response effects were present for high versus low dose ICS for several outcomes (Box 2 A-D).
The NNT to prevent withdrawal because of worsening asthma decreased with increasing doses of fluticasone (Box 3). Treatment of three people with fluticasone 100 μg daily, or treatment of two people with 500 μg of fluticasone daily, was able to prevent one person developing a significant deterioration in asthma control. These data indicate a relatively flat dose–response curve for use of ICS in asthma.
ICS therapy also led to a significant increase in the side effects of hoarseness/dysphonia and oral candidiasis for all doses up to 500 μg daily. There was a trend for more sore throats to be reported for doses < 200 μg/day and a significant increase in sore throats for doses of 500 μg/day. The NNH for hoarseness/dysphonia at 200 μg fluticasone daily was 131, whereas the NNH reduced markedly to 23 (15–52) for a daily dose of 500 μg of fluticasone (Box 3) (ie, for every 23 people treated with fluticasone 500 μg daily, one person developed clinically significant hoarseness/dysphonia). Similarly, the NNH for oral candidiasis was 61 at 200 μg fluticasone daily, and this reduced to 21 (14–46) for fluticasone 500 μg/day (Box 3) (ie, for every 21 people treated with fluticasone 500 μg daily, one person developed oral candidiasis).
No differences were reported in plasma cortisol levels in doses up to 500 μg per day. In one study (good quality) fluticasone 1000 μg/day was associated with a significantly lower plasma cortisol level, but no difference was reported in the other studies.
Box 4 shows the benefits and harm with increasing fluticasone doses. The efficacy curve for different doses of fluticasone is relatively flat. As fluticasone doses increased, there was little gain in terms of efficacy; however, the NNH for oral candidiasis reduced from every 90th patient suffering oral candidiasis with fluticasone 100 μg to every sixth patient with fluticasone 2000 μg.
We present the results of an evidence-based analysis of the efficacy and safety of different doses of ICS for asthma, with the results expressed in clinically meaningful terms — the number needed to treat and the number needed to harm. The results confirm that, in asthma, ICS are highly efficacious, with NNT ranging between 2 and 3 patients. The results also clearly show that the dose–response curve for ICS in asthma is relatively flat, with little difference seen between doses of 100 μg and 1000 μg of fluticasone per day. In terms of efficacy, there appears to be little to be gained from using higher doses of ICS in most people with asthma. While some clinical markers improve with dose escalation, the dose–response curve is relatively flat.
In contrast, the dose–response curve for side effects is steep. Side effects are relatively rare at low doses of ICS, with an NNH for fluticasone 100 μg per day of 131. With increasing doses, the side effect rate progressively increases. At 2000 μg fluticasone daily, the NNH drops to 6. This contrasts with an NNT of 2 indicating a very narrow margin of safety. When these data are displayed graphically (Box 4), with the relevant portion of the 95% CI for each parameter, the gap (unshaded area) represents the safety margin associated with ICS use in asthma. This clearly shows that the main effect of increasing ICS dose in asthma is to increase side effects, with little additional benefit to the patient.
Based on the available evidence, the use of lower doses of ICS would be associated with fewer side effects without loss of efficacy. The results question the current practice in Australia — the widespread use of high-dose ICS.
1: How to calculate the number needed to treat (NNT). For example, for fluticasone 100 μg daily, the number of patients required to receive an intervention to prevent one case of deteriorating asthma. Data obtained from reference 10.
Identify the outcome |
|
Withdrawal due to worsening asthma |
|
Identify the absolute event rate |
|
Control group = 283/496 |
In the control group 283 of 496 subjects withdrew due to worsening asthma, compared with 110 of 507 in the group treated with fluticasone 100 μg daily |
Treatment group = 110/507 |
|
Calculate the absolute risk reduction (ARR) |
|
ARR = 283/496 – 110/507 = 0.35 |
The absolute risk reduction is 0.35. Fluticasone treatment leads to a 35% reduction in the risk of deteriorating asthma |
Calculate number needed to treat (NNT) |
|
NNT = 1/ARR = 1/0.35 = 2.9 |
Take the inverse of the ARR. This is the number of people who need to be treated with fluticasone 100 μg to prevent one case of deteriorating asthma |
2: Efficacy of different doses of fluticasone in asthma
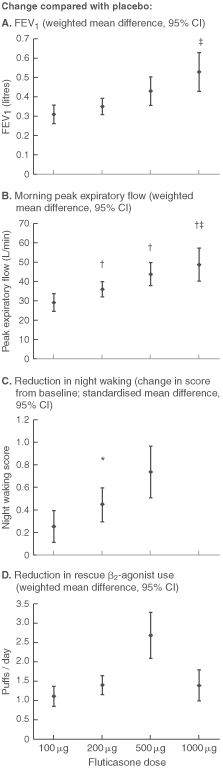
Results are weighted mean difference or standardised mean difference with 95% CI, obtained from meta-analyses of randomised controlled trials.10 * P < 0.05 v 50 μg. † P < 0.05 v 100 μg. ‡ P < 0.05 v 200 μg.11
3: Efficacy and side effects of increasing doses of fluticasone in asthma
|
NNT (95% CI)* |
NNH (95% CI)† |
|||||||||
Fluticasone dose (μg/day) |
Withdrawal because of worsening asthma |
Hoarseness or dysphonia |
Oral candidiasis |
||||||||
100 |
2.9 (2.4–3.4) |
152 (40–1139) |
90 (27–746) |
||||||||
200 |
2.4 (2.2–2.8) |
131 (50–417) |
61 (22–255) |
||||||||
500 |
2.0 (1.7–2.3) |
23 (15–52) |
21 (14–46) |
||||||||
1000 |
2.1 (1.8–2.4) |
17 (11–35) |
23 (14–75) |
2000 |
— |
11 (6–100) |
6 (4–17) |
||||
|
* NNT = Number needed to treat to prevent one additional person withdrawing because of exacerbation of asthma. † NNH = Number needed to treat to prevent one additional person developing side effects. |
|||||||||||
4: Comparison of the relative effects of increasing doses of fluticasone in asthma
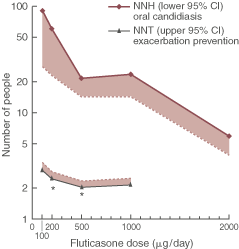
Results for fluticasone are displayed in terms of benefit (number needed to treat; NNT) and harm (number needed to harm; NNH) on a logarithmic scale, with the relevant portion of the 95% CI (shaded area). * Significant heterogeneity present (P < 0.05).
Received 14 August 2002, accepted 10 January 2003
- 1. Suissa S, Ernst P. inhaled corticosteroids: impact on asthma morbidity and mortality. J Allergy Clin Immunol 2001; 107: 937-944.
- 2. National Asthma Council. Asthma management handbook 2002. Melbourne: NAC, 2002.
- 3. National Institutes of Health, National Heart Lung and Blood Institute Workshop report, 2002. Global strategy for asthma management and prevention. NIH publication No. 02-3659. Available at: http://www.ginasthma.com/ (follow link to GINA documents and resources) (accessed January 2003).
- 4. Holt S, Suder A, Wetherall M, et al. Dose-response relation of inhaled fluticasone propionate in adolescents and adults with asthma: a meta-analysis. BMJ 2001; 323: 253-256.
- 5. Black PN, Lawrence BJ, Goh KH, Barry MS. Differences in the potencies of inhaled steroids are not reflected in the doses prescribed in primary care in New Zealand. Eur J Clin Pharmacol 2000; 56: 431-435.
- 6. Todd GR, Acerini CL, Buck JJ, et al. Acute adrenal crisis in asthmatics treated with high-dose fluticasone propionate. Eur Respir J 2002; 19: 1207-1209.
- 7. Altman DG, Anderson PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999; 319: 1492-1495.
- 8. National Health and Medical Research Council. A guide to the development, implementation and evaluation of clinical practice guidelines. Canberra: NHMRC, 1999.
- 9. Cochrane Database of Systematic Reviews. The Cochrane Library. Available at: www.nicsl.com.au/cochrane/index.asp (accessed January 2003).
- 10. Adams N, Bestall J, Jones PW. Inhaled fluticasone propionate for chronic asthma. In: The Cochrane Library Issue 1: 2002. CD 003135.
- 11. Adams N, Bestall J, Jones PW. Inhaled fluticasone propionate at different doses for chronic asthma. In: The Cochrane Library Issue 1: 2002. CD 003534.
- 12. Adams N, Bestall J, Jones PW. Budesonide for chronic asthma in children and adults. In: The Cochrane Library Issue 1: 2002. CD 003274.
- 13. Adams N, Bestall J, Jones PW. Inhaled budesonide at different doses for chronic asthma. In: The Cochrane Library Issue 1: 2002. CD 003271.
- 14. Adams N, Bestall J, Jones PW. Inhaled beclomethasone versus placebo for chronic asthma. In: The Cochrane Library Issue 1: 2002. CD 002738.
- 15. Adams N, Bestall J, Jones PW. Inhaled beclomethasone at different doses for long-term asthma. In: The Cochrane Library Issue 1: 2002. CD 002879.





Abstract
Objective: To define the evidence for doses of inhaled corticosteroids in asthma and describe this in clinically meaningful, evidence-based terms.
Data source: Cochrane Database of Systematic Reviews.
Study selection and data extraction: We identified systematic reviews of randomised controlled trials of dosing of inhaled corticosteroids in asthma. Data on efficacy and safety of different doses were extracted from meta-analyses and summarised as the number needed to treat (NNT) and number needed to harm (NNH).
Data synthesis: Inhaled corticosteroids were highly efficacious, with a relatively flat dose–response curve. Three patients needed to be treated with fluticasone 100 μg daily to prevent worsening asthma (NNT 3), and for fluticasone 1000 μg the NNT was 2.1 patients. The dose–response curve for side effects was steep. For a dose of fluticasone 100 μg, oral candidiasis developed in one of every 90 subjects treated (NNH 90). In contrast, the NNH for fluticasone 1000 μg and 2000 μg daily were 23 and 6, respectively.
Conclusion: Level 1 evidence supports the use of low-dose inhaled corticosteroids in asthma. Clinicians should review doses of inhaled corticosteroids used for treating patients with asthma.