An average of 5000 cases of Ross River virus (RRV) disease are notified each year in Australia.1 This disease presents typically with arthralgia and arthritis.2 Recent studies suggest that symptoms may persist for several years, with over half of patients surveyed complaining of joint pain 12 or more months after onset or diagnosis.3-5 However, these reports are inconsistent with earlier descriptions of RRV disease, possibly because of differences in methods or biases introduced by retrospective study designs.1
Until the natural history of RRV disease is understood, its public health importance cannot be determined, and clinicians will be unable to offer realistic prognoses to their patients. Therefore, we undertook a prospective, inception-cohort study of patients with RRV disease. This design is appropriate for describing the evolution of rheumatic manifestations of RRV disease.
Patients were recruited from general practices in the local government areas of Cairns, Douglas, Mareeba and Atherton in north Queensland during January to May 1998.
We notified all general practitioners in the area of the study by letter, visits (by D H) and a presentation at a continuing medical education session, and encouraged them to refer patients with acute RRV disease for inclusion in the study. In addition, Queensland Health's Tropical Public Health Unit and pathology laboratories in Cairns were asked to report patients with a positive RRV IgM test.
Patients were eligible for the study if they:
were positive for RRV IgM; and
had symptom onset between 1 January and 31 May 1998; and
had resided in the study area for one month before onset; and
the patient and his or her GP consented to their involvement in the study.
When possible, diagnosis was confirmed by IgG seroconversion. Some patients also had serological tests for Barmah Forest virus infection (which can cause a similar illness to RRV disease and false-positive IgM results) and dengue (which was epidemic in Cairns during the study period).1,6-9
The study was approved by the University of Queensland Medical Research and Cairns Base Hospital ethics committees.
A medical practitioner trained in rheumatological assessment (D H) reviewed all patients in their homes as soon as possible after disease onset and on two subsequent occasions.
A clinical history was taken using a standard questionnaire. Joint tenderness on palpation was graded (I: patient complains of pain; II: patient complains of pain and winces; III: patient complains of pain, winces, and withdraws the joint; IV: patient does not allow palpation).10
Statistical analysis was performed using the computer program SPSS.11 For inferences on means and proportions, we used t and χ2 tests. Age and sex distribution were compared between study participants and all notified cases of RRV disease (data obtained from local pathology laboratories and the Tropical Public Health Unit) using a z test on a single mean and a χ2 goodness-of-fit test, respectively.
To evaluate the effect of time since onset on the risk of having more joints affected with pain (of the five most commonly involved), we used ordinal logistic regression with the number of joints affected as the outcome and time since diagnosis as the explanatory variable. To adjust for confounding, age and sex were included. The proportional odds assumption was tested using the likelihood ratio method.12 Because observations were repeated on individuals, a generalised estimating equation approach, with robust estimation of variances, was used to account for the correlated nature of the data using STATA.13
One hundred and twenty-nine patients were notified with a positive RRV IgM result within the study period and area, and 57 (44%) were recruited for the study. The most common reason for non-inclusion was lack of consent from either the patient or treating doctor. Ten patients refused follow-up reviews or were unable to be contacted, while 47 of the 57 were reviewed three times and constituted the inception cohort. Those reviewed three times were older, more likely to be women, tertiary educated and to work as professionals, managers or administrators than those reviewed once or twice, but none of these differences was statistically significant (Box 1).
The 47 participants in the study were from the local government areas of Cairns (34), Mareeba (10), Douglas (2) and Atherton (1). Age did not differ significantly between the 47 study participants and notified patients who were not included in the study (including the 10 reviewed once or twice only). Sex distribution also did not differ significantly.
Thirteen of the 47 cohort cases were confirmed by IgG seroconversion. Tests for Barmah Forest and dengue viruses were undertaken for 24 and 22 of the 47, respectively, all with negative results.
Eight of the 47 participants reported pre-existing joint disease: osteoarthritis (4), gout (1), psoriatic arthritis (1), and other arthritides (2). Other pre-existing diseases included asthma (7), hypertension (3), diabetes (1) and chronic fatigue syndrome (1).
Mean times from disease onset to first, second and third reviews were 1.1 months (range, 0.2 to 3.6), 2.4 months (range, 1.2 to 5.0), and 3.6 months (range, 2.3 to 6.5 months), respectively.
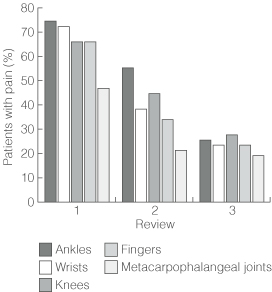
Prevalence of joint symptoms is shown in Box 2. Joint pain was the most common symptom at all three reviews (98%, 92% and 68% of patients, respectively), followed by joint stiffness, myalgia and joint swelling. Prevalence of each symptom decreased at successive reviews. However, at third review, joint pain was still present in seven of the eight participants with pre-existing joint disease (88%) and 25 of the 39 without (64%) (Yates' corrected χ2 = 0.77; P = 0.38). Joint pain most commonly involved ankle, wrist, knee, finger and metacarpophalangeal joints (Box 3), but decreased in prevalence over time at each of these joint types (Box 4).
Abnormal findings on physical examination at first review included joint effusion (2 patients), synovial thickening (3), joint swelling and heat (4), enthesopathy (2) and plantar fasciitis (1). Prevalence of abnormalities typically decreased at subsequent reviews. Prevalence of normal findings and grade I tenderness at different joints is shown in Box 2. Few participants had tenderness greater than grade I, with little change in prevalence throughout. At the final review, between 94% (ankle joints) and 100% (metacarpophalangeal, wrist, elbow, metatarsophalangeal and interphalangeal joints of toes) had normal findings and grade I tenderness.
Use of anti-inflammatory drugs is shown in Box 2. At first review, 29 participants were taking non-steroidal anti-inflammatory drugs (NSAIDs), and three were using oral (2) or injected (1) corticosteroids, the last a patient with pre-existing osteoarthritis. Use of NSAIDs decreased with successive reviews.
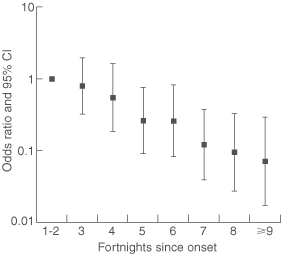
The association between number of joint types with pain and time since disease onset was determined by ordinal logistic regression adjusted for age and sex (Box 5). Odds ratios for having pain in more joint types than within the first four weeks after onset decreased significantly with time (P < 0.001). The odds ratio associated with a change in the number of joints with pain per day since disease onset was 0.98 (95% CI, 0.97–0.99), and per fortnight was 0.72 (95% CI, 0.61–0.85). Consequently, there was a 2% decrease per day in the risk of having more joints with pain than at the first visit and a 28% decrease per fortnight.
The proportional odds assumption was valid (likelihood ratio statistic [G] = 2.58; df = 4; P = 0.63), indicating that ordinal logistic regression was appropriate for analysis of these data.
Our results show that acute RRV disease is not a trivial illness, with most patients initially reporting joint pain. However, in contrast to some previous studies,3-5 we found that symptoms steadily resolved over three to six months.
Our study has the advantage of repeated clinical review by a single investigator and exclusion of other diagnoses, such as Barmah Forest virus infection and dengue. This, along with the high specificity of the tests for RRV IgM and IgG (96.5% and 97.6%, respectively14) (Dr Ming Qiao, Clinical Microbiologist, Institute of Medical and Veterinary Science, personal communication, 2001), make misdiagnosis unlikely. In earlier studies that have found more persistent symptoms,3-5 diagnoses are likely to have been almost entirely presumptive, without exclusion of other diagnoses.1 In such studies, up to 20% of cases may have been misdiagnosed.15
The major weaknesses of our study are its modest sample size and the absence of a control group. The latter prevents absolute quantification of the morbidity attributable to RRV disease, but data from other reports and population surveys allow some inferences. Arthralgia and arthritis are common in all populations. In Australia, 16.7% of GP encounters are for musculoskeletal complaints.16 In the United States, the prevalence of self-reported arthritis in 1997 was 16.1%,16 while in Spain 3.7% of patients in a general paediatric outpatient clinic presented with arthralgia.18 Consequently, joint pain due to causes other than RRV disease might account for some of the residual symptoms present in our cohort at final review.
Although we cannot exclude selection bias, we found that our cohort was representative of all notified patients in terms of age and sex. However, we cannot assume that notified patients who did not participate in the study had similar symptoms to participants. Nevertheless, biases in our study are likely to have been substantially less than those in retrospective studies that rely on self-reported symptoms.3-5
The course of rheumatic manifestations in RRV disease seen in our study differs from that in previous reports. It justifies GPs giving optimistic prognoses, as most patients are pain-free within three to six months of disease onset.
As RRV disease causes a well-defined syndrome2 and involves specific joints,1 it is inappropriate to ascribe symptoms that do not fit this syndrome to RRV disease. We recommend specific questioning and recording of pain in the most commonly affected joints (ankle, wrist, fingers, knee and metacarpophalangeal joints) as an index of disease activity. Alternative diagnoses should be considered for patients with symptoms that last longer than six months or without involvement of the commonly affected joints.
Detailed assessment of the overall economic impact of RRV is needed. Also, as the cost of serological testing for RRV is considerable,1 we need to know the sensitivity and specificity of clinical diagnosis. The yield and costs of different diagnostic strategies could then be determined.
1: Comparison between patients reviewed three times (study participants) and those reviewed once or twice
Characteristic |
1–2 reviews (n = 10) |
3 reviews (n = 47) |
P |
||||||||
Mean age in years (SD) |
34.3 (10.3) |
39.5 (11.3) |
0.19* |
||||||||
Women |
4 (40%) |
25 (53%) |
0.68† |
||||||||
Professionals, managers and administrators |
1 (10%) |
11 (23%) |
0.61† |
||||||||
Tertiary educated |
1 (10%) |
12 (26%) |
0.52† |
||||||||
* t test. † Yates' corrected χ2 test. |
|||||||||||
2: Frequency of joint symptoms and tenderness and use of anti-inflammatory drugs in 47 patients with Ross River virus disease
Number of patients |
|||||||||||
Review 1
|
Review 2
|
Review 3
|
|||||||||
Symptom |
|||||||||||
Joint pain |
46 (98%) |
43 (92%) |
32 (68%) |
||||||||
Joint stiffness |
42 (89%) |
34 (72%) |
17 (36%) |
||||||||
Myalgia |
28 (60%) |
18 (38%) |
12 (26%) |
||||||||
Joint swelling |
25 (53%) |
13 (28%) |
5 (11%) |
||||||||
Normal or grade I joint tenderness† |
|||||||||||
Feet |
42 (89%) |
44 (94%) |
46 (98%) |
||||||||
Metatarsophalangeal joints |
43 (92%) |
47 (100%) |
47 (100%) |
||||||||
Ankles |
44 (94%) |
44 (94%) |
44 (94%) |
||||||||
Fingers (proximal interphalangeal joints) |
44 (94%) |
44 (94%) |
45 (96%) |
||||||||
Toes |
44 (94%) |
46 (98%) |
47 (100%) |
||||||||
Knees |
44 (94%) |
47 (100%) |
47 (100%) |
||||||||
Fingers (distal interphalangeal joints) |
45 (96%) |
46 (98%) |
45 (96%) |
||||||||
Elbows |
45 (96%) |
46 (98%) |
45 (96%) |
||||||||
Metacarpophalangeal joints |
47 (100%) |
45 (96%) |
47 (100%) |
||||||||
Wrists |
47 (100%) |
46 (98%) |
47 (100%) |
||||||||
Drug use |
|||||||||||
NSAID alone |
29 (62%) |
15 (32%) |
7 (15%) |
||||||||
Corticosteroid alone (oral or injected) |
3 (6%) |
1 (2%) |
0 |
||||||||
NSAID plus oral corticosteroid |
0 |
1 (2%) |
1 (2%) |
||||||||
NSAID = non-steroidal anti-inflammatory drug. * Mean months after symptom onset. † Joint tenderness was defined as grade I if patient complained of pain on palpation of joint, grade II if patient also winced, grade III if patient also withdrew joint, and grade IV if patient did not allow palpation. |
|||||||||||
3: Prevalence of joint pain at first review* of 47 patients with Ross River virus disease
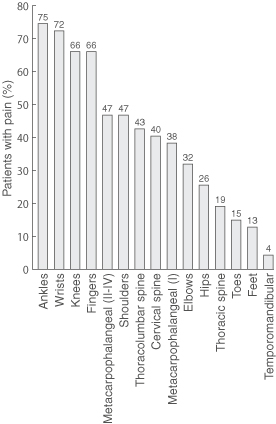
* First review was at 1–15 weeks after symptom onset. Patients were asked about pain in the preceding week.
Received 24 September 2001, accepted 6 June 2002
- David Harley1
- David Bossingham2
- David M Purdie3
- Nirmala Pandeya4
- Adrian C Sleigh5
- 1 Developmental Disability Unit, School of Population Health, University of Queensland, Mater Hospital, Brisbane, QLD.
- 2 Abbott Street, Cairns, QLD.
- 3 Queensland Institute of Medical Research, Royal Brisbane Hospital, Brisbane, QLD.
- 4 University Department of Rural Health, Faculty of Health Sciences, University of Tasmania, Launceston, TAS.
We thank Ms Jenny Day (University Of Queensland) and Ms Andrea Mylonas (Queensland Institute of Medical Research), Professor Gail Williams (University Of Queensland), Queensland Medical Laboratory, Sullivan and Nicolaides Pathology, Cairns Base Hospital Pathology Laboratory, and Cairns Division of General Practice for their assistance. We also thank Dr Jeff Hanna, Dr Ross Spark, Ms Fiona Tulip and other staff at the Tropical Public Health Unit, Cairns, and the general practitioners and patients who participated. The National Health and Medical Research Council, the Royal Australian College of General Practitioners and the Australian Centre for International and Tropical Health and Nutrition, University of Queensland, provided financial support. The study was part of a PhD thesis completed by D H in the Australian Centre for International and Tropical Health and Nutrition, University of Queensland.
None identified.
- 1. Harley D, Sleigh A, Ritchie S. Ross River virus transmission, infection and disease: a cross-disciplinary review. Clin Microbiol Rev 2001; 14: 909-932.
- 2. Fraser JRE. Epidemic polyarthritis and Ross River Virus disease. Clin Rheum Dis 1986; 12: 369-388.
- 3. Condon RJ, Rouse IL. Acute symptoms and sequelae of Ross River virus infection in South-Western Australia. A follow-up study. Clin Diagn Virol 1995; 3: 273-284.
- 4. Selden SM, Cameron AS. Changing epidemiology of Ross River virus disease in South Australia. Med J Aust 1996; 165: 313-317.
- 5. Westley-Wise VJ, Beard JR, Sladden TJ, et al. Ross River virus infection on the North Coast of New South Wales. Aust N Z J Public Health 1996; 20: 87-92.
- 6. Flexman JP, Smith DW, Mackenzie JS, et al. A comparison of the diseases caused by Ross River virus and Barmah Forest virus. Med J Aust 1998; 169: 159-163.
- 7. Rich G, McKechnie J, McPhan I, Richards B. Laboratory diagnosis of Ross River virus infection. Commun Dis Intell 1993; 17: 208-209.
- 8. Mackenzie JS, Broom AK, Calisher CH, et al. Diagnosis and reporting of arbovirus infections in Australia. Arbovirus Res Aust 1993; 6: 89-93.
- 9. Hanna JN, Ritchie SA, Phillips DA, et al. An epidemic of dengue 3 in far north Queensland, 1997–1999. Med J Aust 2001; 174: 178-182.
- 10. Talley NJ, O'Connor SO. Clinical examination. 2nd ed. London: MacLennan and Petty, 1992.
- 11. SPSS [computer program]. Release 10.0.5. Chicago, Ill: SPSS Inc, 1989-1999.
- 12. Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York: John Wiley and Sons, 2000.
- 13. Stata statistical software [computer program]. Release 7.0. College Station, TX: Stata Corporation, 2001.
- 14. Lucas RE, Qiao M. A case of encephalitis in central Australia due to Ross River virus? Aust N Z J Med 1999; 29: 268-270.
- 15. Lloyd A, Hickie I, Vollmer-Conna U, et al. Characterisation of prolonged ill health following Ross River virus infection: a prospective cohort study. Arbovirus Res Aust 2000; 8: 236.
- 16. Britt H, Sayer GP, Miller GC, et al. General practice activity in Australia 1998-99. Canberra: Australian Institute of Health and Welfare, 1999.
- 17. Prevalence of arthritis — United States, 1997. MMWR Morb Mortal Wkly Rep 2001; 50: 334-336.
- 18. de Inocencio J. Musculoskeletal pain in primary pediatric care: analysis of 1000 consecutive general pediatric clinic visits. Pediatrics 1998; 102: E63.





Abstract
Objective: To describe the natural history of rheumatic manifestations of Ross River virus (RRV) disease.
Design: Prospective longitudinal clinical review.
Setting: North Queensland local government areas of Cairns, Douglas, Mareeba and Atherton during January to May 1998.
Participants: General practice patients diagnosed with RRV disease on the basis of symptoms and a positive RRV IgM result.
Main outcome measures: Rheumatic symptoms and signs assessed as soon as possible after disease onset and on two subsequent occasions (up to 6.5 months after onset).
Results: 57 patients were recruited, 47 of whom were reviewed three times (at means of 1.1, 2.4 and 3.6 months after disease onset). Results are reported for these 47: 46 (98%) complained of joint pain at first review, with the ankles, wrists, fingers, knees and metacarpophalangeal joints (II–IV) most commonly involved. Prevalence of joint pain decreased progressively on second and third reviews, both overall (92% and 68% of patients, respectively), and in the five joints most commonly affected. The prevalence of other common rheumatic symptoms and signs, and use of non-steroidal anti-inflammatory drugs, also progressively declined over the three reviews.
Conclusions: Earlier studies may have overestimated the prevalence and duration of symptoms in RRV disease. Progressive resolution over 3–6 months appears usual.